Korean J Hematol.
2007 Sep;42(3):206-215. 10.5045/kjh.2007.42.3.206.
The Association between Cytogenetic Abnormalities and Clinical Outcomes Based on Prognostic Factors of the Children Cancer Group (CCG) in Pediatric Patients with Acute Leukemia: Two Institutional Retrospective Studies
- Affiliations
-
- 1Department of Pediatrics, Hanyang University College of Medicine, Seoul, Korea. cord@hanyang.ac.kr
- 2Department of Laboratory Medicine, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2305195
- DOI: http://doi.org/10.5045/kjh.2007.42.3.206
Abstract
-
BACKGROUND: We investigated the incidence of cytogenetic abnormalities as well as the correlation of the cytogenetic abnormalities and clinical outcomes based on the prognostic factors of the Children Cancer Group (CCG) in children with acute leukemia.
METHODS
We retrospectively reviewed the cytogenetic studies and clinical data from 99 children that were diagnosed with acute leukemia and treated with CCG regimens in two institutions. A conventional cytogenetic analysis was performed.
RESULTS
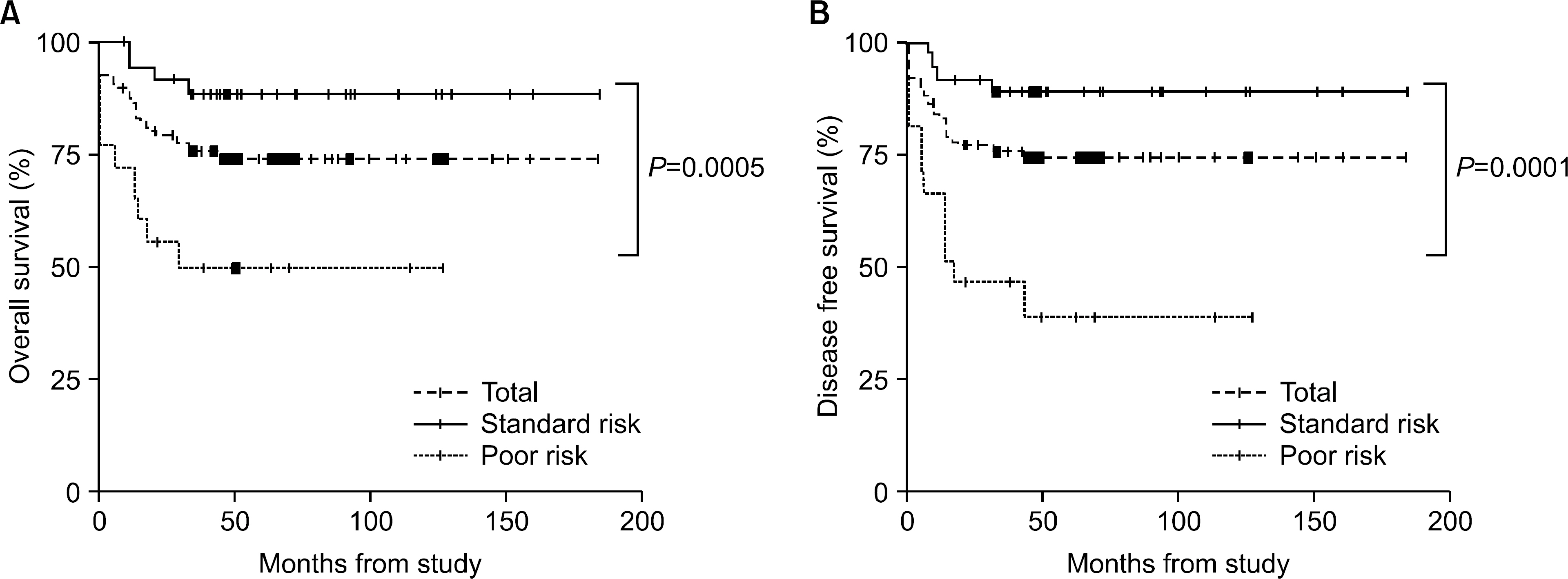
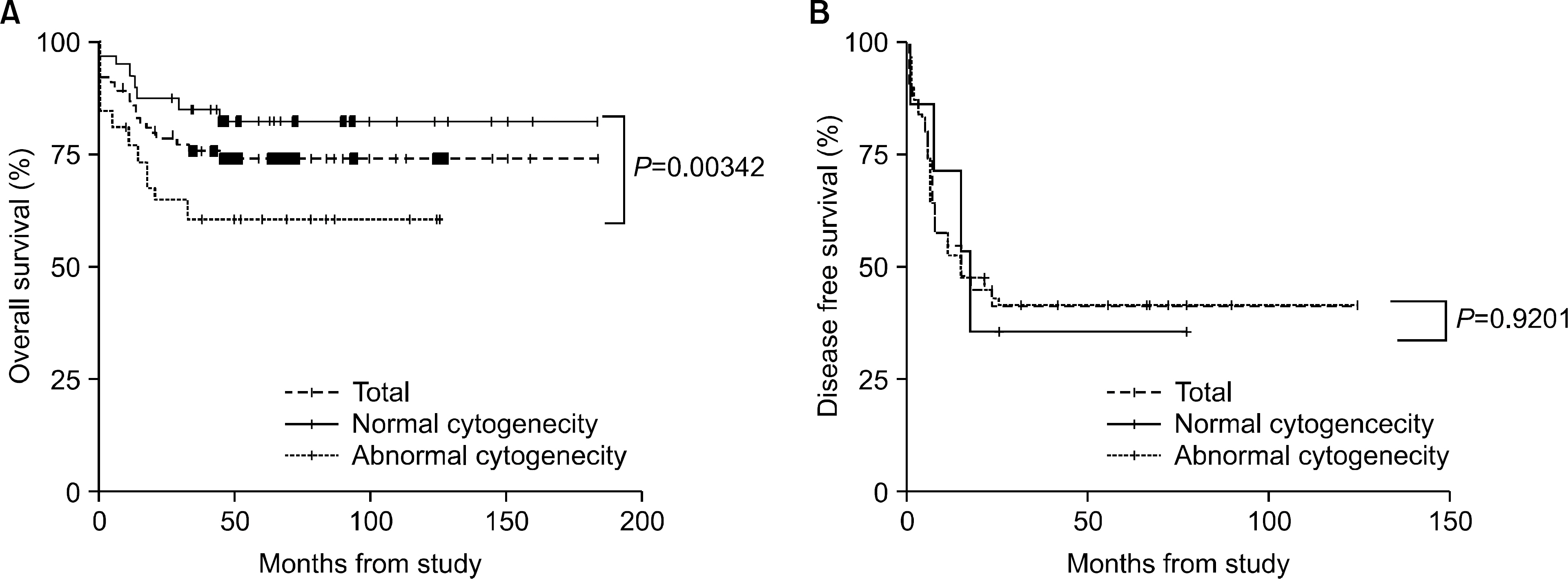
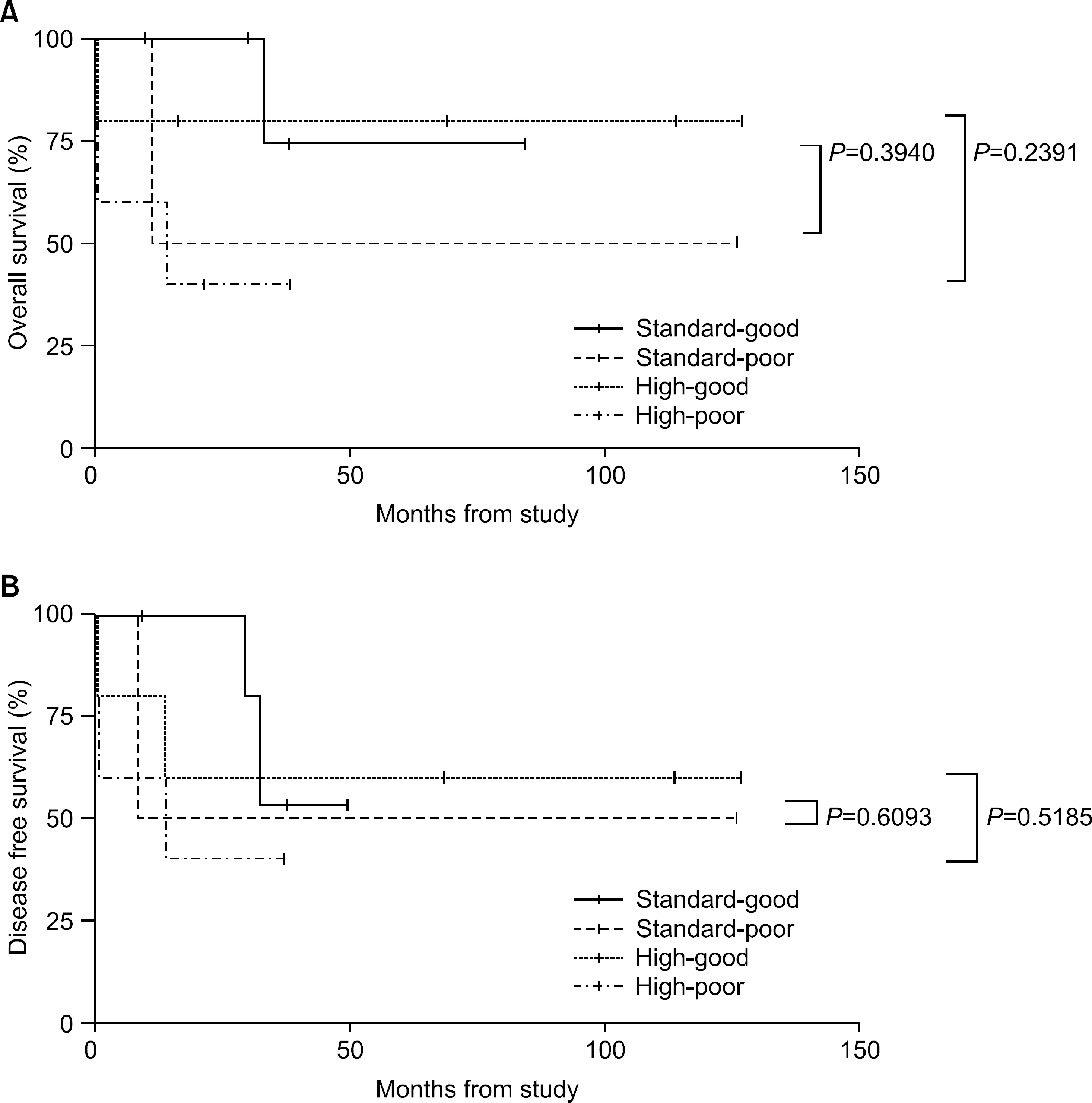
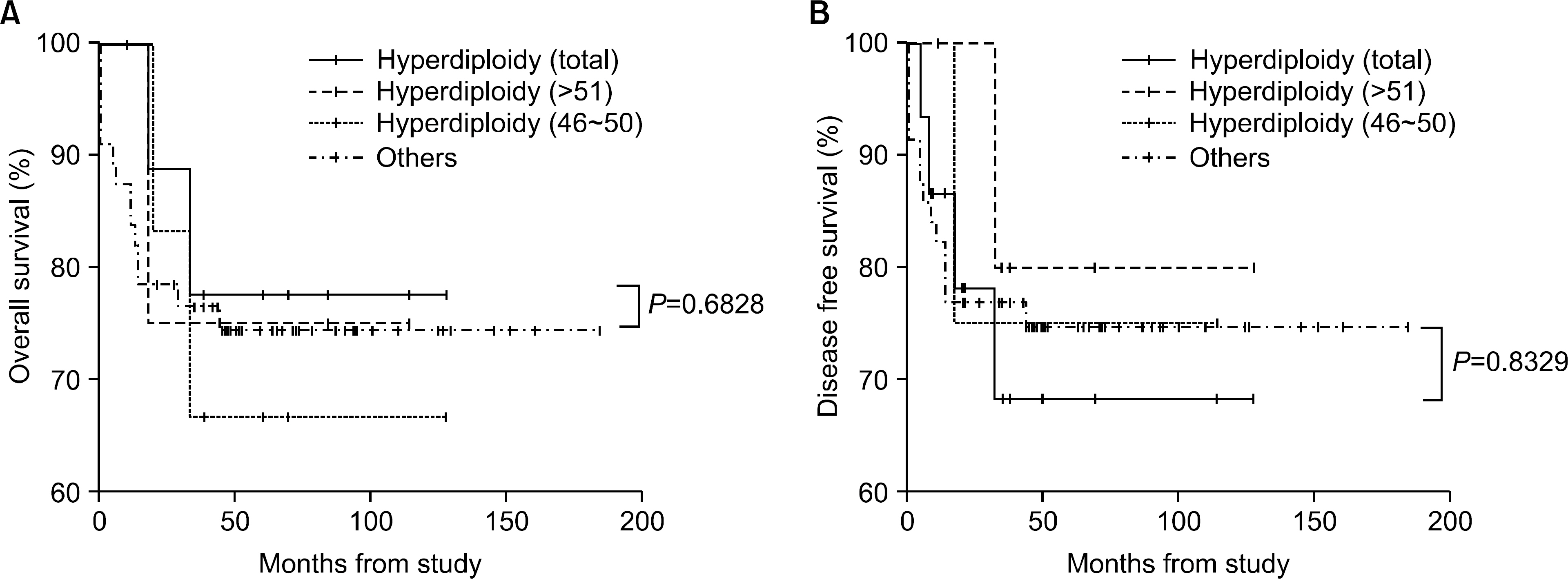
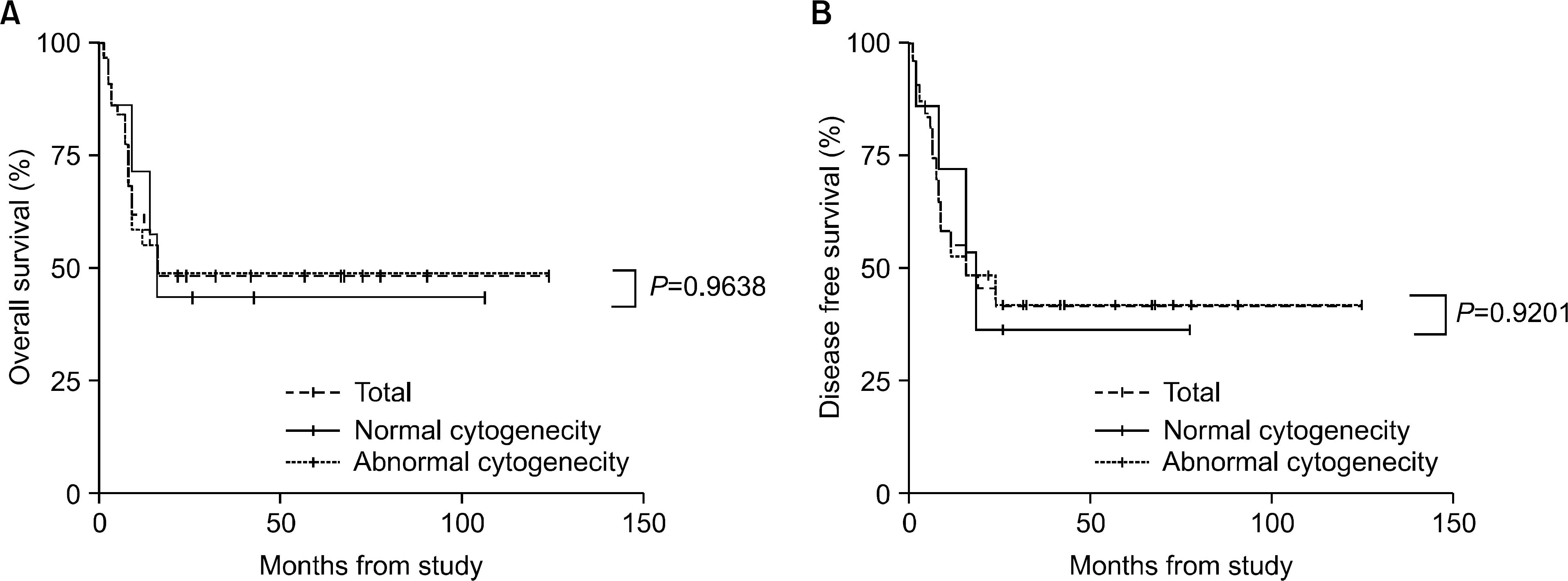
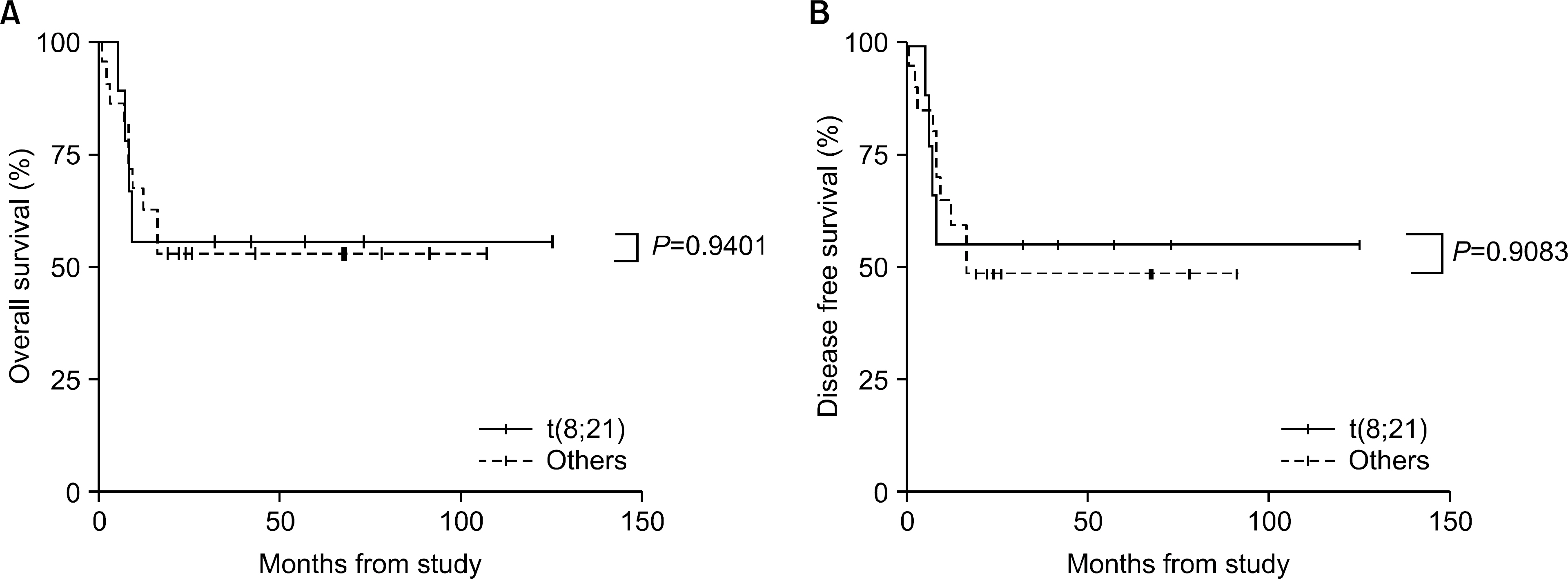
The incidence of cytogenetic abnormalities was 51 (51.5%) in 99 patients, and 27 (39.7%) in acute lymphoblastic leukemia (ALL) patients and 24 (77.4%) in acute myelogenous leukemia (AML) patients. The most frequent cytogenetic abnormality was hyperdiploidy and t(8:21) in the ALL and AML patients, respectively. The overall survival rate (OS)/disease free survival rate (DFS) of the ALL patients was 74.0%/73.9%. The OS/DFS of the standard risk group (88.8%/85.2%) was significantly higher than that of the high-risk group (49.4%/39.3%) in the ALL patients (P=0.0005/P<0.0001). There was no significant difference in the survival rates according to the type of cytogenetic abnormalities among the ALL patients for the standard/high risk groups, based on the CCG prognostic factors. The OS/DFS of the AML patients were 43.4% and 41.7%, respectively, without significant differences of the survival rates according to the type of chromosomal abnormalities.
CONCLUSION
There were significant differences of OS/DFS based on the risk groups in ALL patients when evaluated with the CCG prognostic factors (standard/high) and chromosomal abnormalities (good/ poor), respectively. However, there was no significant correlation between type of cytogenetic abnormalities and clinical outcomes based on the CCG prognostic factors in children with ALL as well as with AML.
Keyword
MeSH Terms
Figure
Reference
-
1). Mrozek K., Heerema NA., Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004. 18:115–36.2). Johansson B., Mertens F., Mitelman F. Geographic hetereogeneity of neoplasia-associated chromosome aberrations. Genes Chromosomes Cancer. 1991. 3:1–7.3). Heerema NA., Nachman JB., Sather HN, et al. Hypodiploidy with less than 45 chromosomes confers adverse risk in childhood acute lymphoblastic leukemia: a report from the Children's Cancer Croup. Blood. 1999. 94:4036–45.4). Chessels JM., Swansbury GJ., Reeves B., Bailey CC., Richards SM. Cytogenetics and prognosis in childhood lymphoblastic leukaemia: results of MRC UKALL X. Medical research council working party in childhood leukaemia. Br J Haematol. 1997. 99:93–100.5). Berger R., Flandrin G., Bernheim A, et al. Cytogenetic studies on 519 consecutive de novo acute nonlymphocytic leukemias. Cancer Genet Cytogenet. 1987. 29:9–21.
Article6). Raimondi SC., Chang MN., Ravindranath Y, et al. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a cooperative pediatric oncology group study-POG 8821. Blood. 1999. 94:3707–16.7). Ha JS., Ryoo NH., Jeon DS., Kim JR., Kim YJ. The Cytogenetic analysis of leukemia patient; a study of 515 cases. Korean J Hematol. 2003. 38:8–14.8). Han JY., Kim KH., Lee YH., Kim JS., Kim HJ., Lee EY. Analysis of clonal chromosome abnormalities in acute myeloid leukemia at diagnosis, in remission, and in relapse. Korean J Clin Pathol. 2000. 20:1–6.9). Ki MN., Kim EA., Lyu CJ., Yang CH., Kom KY., Choi JR, et al. Chromosomal analysis in childhood leukemia. Korea J Pediatr Hematol Oncol. 2001. 8:231–7.10). Shaffer LG., Tommerup N. An International System for Human Cytogenetic Nomenclature. Basel, Switzerland: S.karger. 2005.11). Ma SK., Wan TS., Chan LC. Cytogenetics and molecular genetics of childhood leukemia. Hematol oncol. 1999. 17:91–105.
Article12). PuiC-H. Crist WM., Look AT. Biology and clinical significance of cytogenetic abnormalities in childhood acute lymphoblastic leukemia. Blood. 1990. 76:1449–63.
Article13). Trueworthy R., Shuster J., Look T, et al. Ploidy of lymphoblasts is the strongest predictor of treatment outcome in B-progenitor cell acute lymphoblastic leukemia of childhood: a Pediatric Oncology Group study. J Clin Oncol. 1992. 10:606–13.
Article14). Whitehead VM., Vuchich MJ., Lauer SJ, et al. Accumulation of high levels of methotrexate poly-glutamates in lymphoblasts from children with hyperdiploid (greater than 50 chromosomes) B-lineage acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood. 1992. 80:1316–23.
Article15). Ito C., Kumagai M., Manabe A, et al. Hyperdiploid acute lymphoblastic leukemia with 51 to 65 chro-mosoines: a distint biological entity with a marked propensity to undergo apoptosis. Blood. 1999. 93:315–20.16). Fletcher JA., Kimball VM., Lynch E, et al. Prognostic implications of cytogenetic studies in an intensively treated group of children with acute lymphoblastic leukemia. Blood. 1989. 74:2130–5.
Article17). Rubnitz JE., Downing JR., Pui CH, et al. TEL gene rearrangement in acute lymphoblastic leukemia: a new genetic marker with prognostic significance. J Clin Oncol. 1997. 15:1150–7.
Article18). Uckun FM., Pallisgaard N., Hokland P, et al. Expression of TEL-AML1 fusion transcripts and response to induction therapy in standard risk acute lymphoblastic leukemia. Leuk Lymphoma. 2001. 42:41–56.
Article19). Uckun FM., Nachman JB., Sather HN, et al. Poor treatment outcome of Philadelphia chromosome-positive pediatric acute lymphoblastic leukemia despite intensive chemotherapy. Leuk Lymphoma. 1999. 33:101–6.
Article20). Arico M., Valsecchi MG., Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome positive acute lymphoblastic leukemia. N Engl J Med. 2000. 342:998–1006.21). Pui CH., Gaynon PS., Boyett JM, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002. 359:1909–15.
Article22). Uderzo C., Valsecchi MG., Balduzzi A, et al. Allogeneic bone marrow transplantation versus chemotherapy in high-risk childhood acute lymphoblastic leukaemia in first remission. Association Italiana di Ematologia de Oncologia Pediatrica (AIEOP) and the Grouppo Italiano Trapianto di midollo Osseo (GITMO). Br J Haematol. 1997. 96:387–94.23). Sharathkumar A., Saunders EF., Dror Y, et al. Allogeneic bone marrow transplantation vs chemotherapy for children with Philadelphia chromosome-positive acute lymphoblastic leukemia. Bone Marrow Transplant. 2004. 33:39–45.
Article24). Grimwade D., Walker H., Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1612 patients entered into the MRC AML 10 trial. The medical research council adult and children's leukaemia working parties. Blood. 1998. 92:2322–33.25). Byrd JC., Mrozek K., Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002. 100:4325–36.
Article26). Visani G., Bernasconi P., Boni M, et al. The prognostic value of cytogenetics is reinforced by the kind of induction/consolidation therapy in influencing the outcome of acute myeloid leukemia—analysis of 848 patients. Leukemia. 2001. 15:903–9.27). Kolitz JE., George SL., Dodge RK, et al. Consolidation therapy by cytogenetic risk in adults with acute myeloid leukemia (AML) ꠚ 60 years in first complete remission (CR): results of CALGB 9621. Blood. 2001. 98:A–688. abstract.28). Dahl GV., Weinstein HJ. Acute myeloid leukemia in children. Hoffman R, editor. Hematology: basic principles and practice. 4th ed.Florida: Churchill Livingstone;2005. p. 500–8.29). Raimondi SC., Chang M N., Ravindranath Y, et al. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a cooperative pediatric oncology group study-POG 8821. Blood. 1999. 94:3707–16.30). Bloomfield CD. Prognostic factors for selecting curative therapy for adult acute myeloid leukemia. Leukemia. 1992. 6(Suppl 4):65–7.31). Woods WG., Neudorf S., Gold S, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001. 97:56–62.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Pediatric Acute Lymphoblastic Leukemia with Trisomy 5 as a Sole Chromosomal Anomaly: A Prognostic Significance
- Survival of Children with Acute Lymphoblastic Leukemia with Risk Group–Based Protocol Changes: A Single-Center Experience with 460 Patients over a 20-Year Period
- Recent advances in the treatment of pediatric acute leukemia
- Clinical Characteristics of Biphenotypic Acute Leukemia in Childhood: A Single Institutional Experience
- Characteristics and Therapeutic Outcomes of Acute Promyelocytic Leukemia in Children and Adolescents