J Rheum Dis.
2013 Aug;20(4):223-230. 10.4078/jrd.2013.20.4.223.
The Urate-lowering Efficacy and Safety of Febuxostat in Korean Patients with Gout
- Affiliations
-
- 1Department of Internal Medicine, Seoul St. Mary's Hospital, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. ysong@snu.ac.kr
- 3Department of Internal Medicine, Inha University Hospital, Incheon, Korea.
- 4Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Severance Hospital, Seoul, Korea.
- 7Department of Medicine, Hanyang University Medical Center, Seoul, Korea.
- 8Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 9Department of Internal Medicine, Hallym University Sacred Heart Hospital, Pyeongchon, Korea.
- 10Department of Internal Medicine, Gachon Medical School Gil Medical Center, Incheon, Korea.
- 11Department of Biostatistics, Seo Kyeong University, Seoul, Korea.
- KMID: 2297536
- DOI: http://doi.org/10.4078/jrd.2013.20.4.223
Abstract
OBJECTIVE
To compare the urate-lowering efficacy and the safety of febuxostat, allopurinol and placebo in Korean patients with gout for 4 weeks.
METHODS
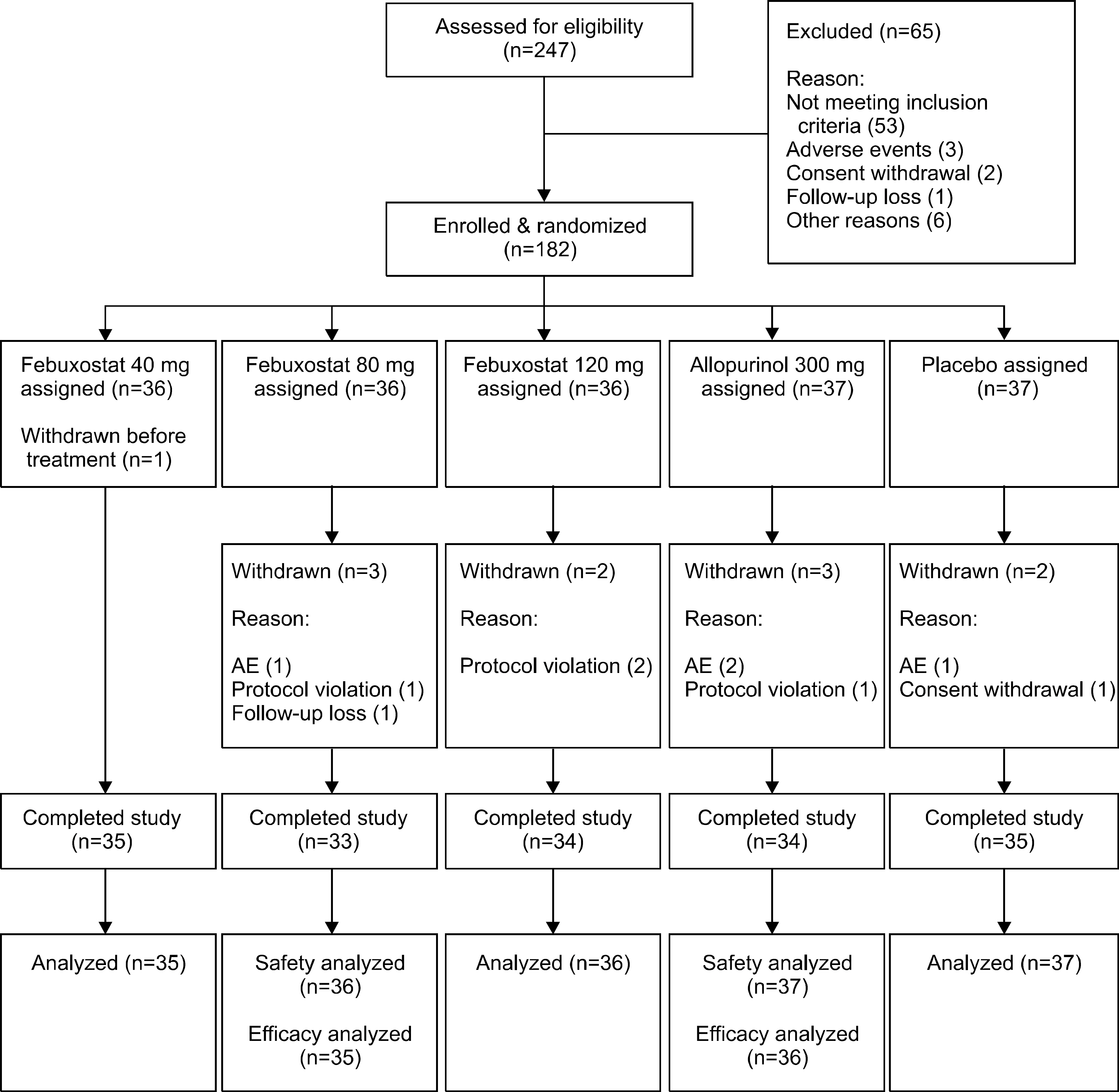
Subjects (n=182) with gout were randomized to febuxostat (40, 80, 120 mg), allopurinol 300 mg, or placebo group. The primary end point was the proportion of subjects whose serum urate concentration fell to less than 6.0 mg/dL after the 4-week treatment.
RESULTS
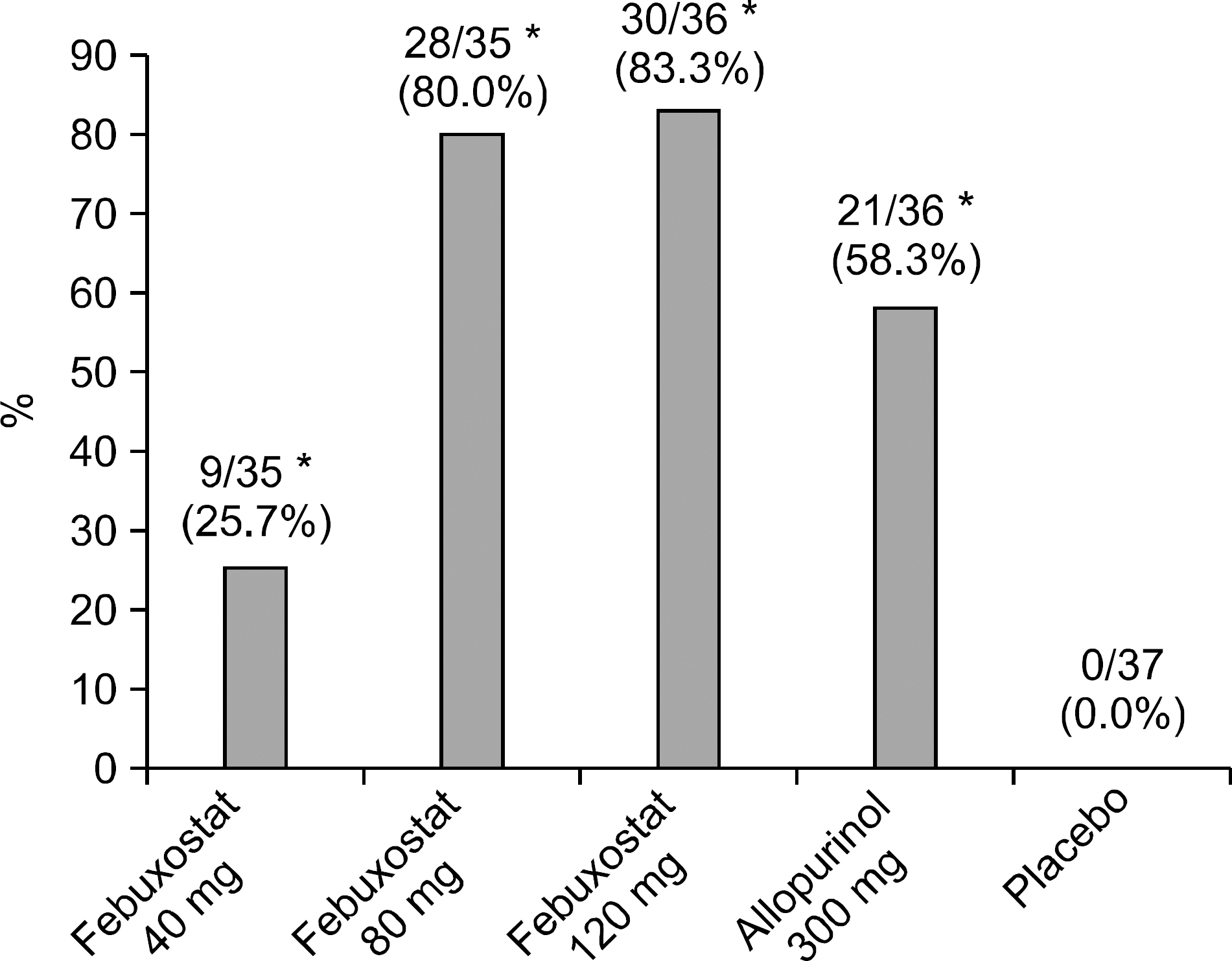
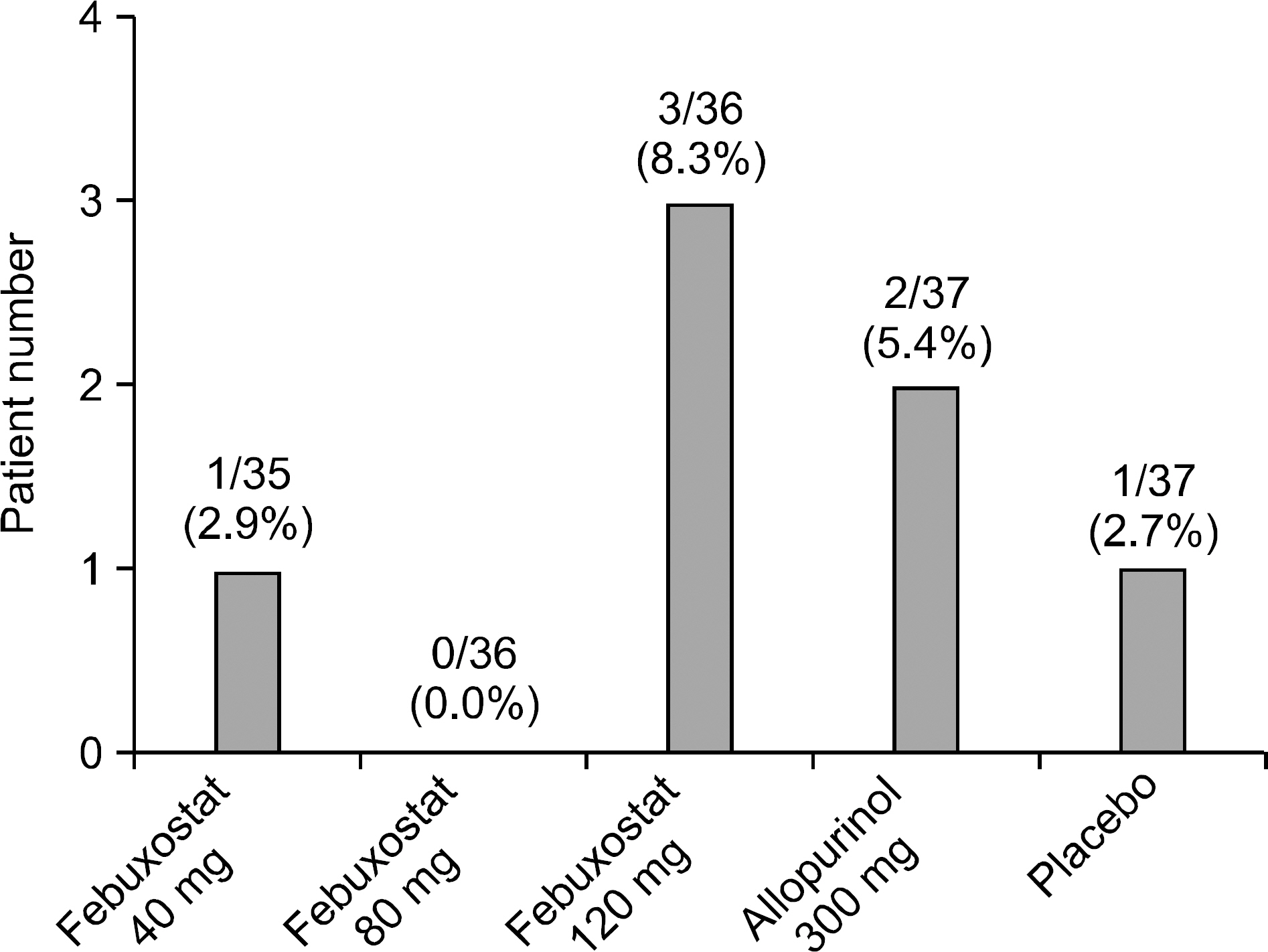
The primary end point was reached at 25.7%, 80.0% and 83.3% of patients receiving 40, 80 and 120 mg of febuxostat, respectively, 58.3% of those receiving 300 mg of allopurinol and none of the placebo (p<0.001: each febuxostat dose or allopurinol group versus placebo group, p=0.0484 and p=0.0196: febuxostat 80 and 120 mg compared with allopurinol, respectively). The number and proportion of subjects who developed adverse events (AEs) were 13 subjects (37%), 14 (39%) and 18 (50%) in the febuxostat of 40, 80 and 120 mg group, respectively, 21 (57%) in the allopurinol 300 mg group and 17 (46%) in the placebo group. No statistically significant differences in the incidence rates of adverse events were observed between the groups. There was no significant difference in gout flare-up incidence.
CONCLUSION
Febuxostat, 80 mg or 120 mg, was more effective than allopurinol (300 mg) or placebo, when lowering the serum urate. The safety of febuxostat and allopurinol was comparable.
Keyword
Figure
Cited by 2 articles
-
The Efficacy and Safety of Febuxostat in Korean Patients with Gout
Sung Won Lee
J Rheum Dis. 2013;20(5):277-279. doi: 10.4078/jrd.2013.20.5.277.Comparative Efficacy and Safety of Febuxostat and Allopurinol in the Treatment of Hyperuricemia: A Bayesian Network Meta-analysis
Gwan Gyu Song, Young Ho Lee
J Rheum Dis. 2015;22(6):356-365. doi: 10.4078/jrd.2015.22.6.356.
Reference
-
References
1. Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010; 6:30–8.
Article2. Terkeltaub R, Zelman D, Scavulli J, Perez-Ruiz F, Lioté F. Gout Study Group: update on hyperuricemia and gout. Joint Bone Spine. 2009; 76:444–6.
Article3. Chohan S, Becker MA. Update on emerging urate-lowering therapies. Curr Opin Rheumatol. 2009; 21:143–9.
Article4. Osada Y, Tsuchimoto M, Fukushima H, Takahashi K, Kondo S, Hasegawa M, et al. Hypouricemic effect of the novel xanthine oxidase inhibitor, TEI-6720, in rodents. Eur J Pharmacol. 1993; 241:183–8.
Article5. Khosravan R, Grabowski BA, Wu JT, Joseph-Ridge N, Vernillet L. Pharmacokinetics, pharmacodynamics and safety of febuxostat, a nonpurine selective inhibitor of xanthine oxidase, in a dose escalation study in healthy subjects. Clin Pharmacokinet. 2006; 45:821–41.
Article6. Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, et al. Selectivity of febuxostat, a novel nonpurine inhibitor of xanthine oxidase/xanthine dehy-drogenase. Life Sci. 2005; 76:1835–47.
Article7. Komoriya K, Osada Y, Hasegawa M, Horiuchi H, Kondo S, Couch RC, et al. Hypouricemic effect of allopurinol and the novel xanthine oxidase inhibitor TEI-6720 in chimpanzees. Eur J Pharmacol. 1993; 250:455–60.
Article8. Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010; 12:R63.
Article9. Becker MA, Schumacher HR, MacDonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol. 2009; 36:1273–82.
Article10. Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008; 59:1540–8.
Article11. Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005; 353:2450–61.
Article12. Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Palo WA, Eustace D, et al. Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty-eight-day, multicenter, phase II, randomized, double-blind, placebo-controlled, dose-response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum. 2005; 52:916–23.
Article13. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977; 20:895–900.
Article14. Gray CL, Walters-Smith NE. Febuxostat for treatment of chronic gout. Am J Health Syst Pharm. 2011; 68:389–98.
Article15. Lee MH, Graham GG, Williams KM, Day RO. A bene-fit-risk assessment of benzbromarone in the treatment of gout. Was its withdrawal from the market in the best in-terest of patients? Drug Saf. 2008; 31:643–65.
Article16. Whelton A, Macdonald PA, Zhao L, Hunt B, Gunaward-hana L. Renal function in gout: longterm treatment effects of febuxostat. J Clin Rheumatol. 2011; 17:7–13.17. Bardin T. Current management of gout in patients un-responsive or allergic to allopurinol. Joint Bone Spine. 2004; 71:481–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Renal safety and urate-lowering efficacy of febuxostat in gout patients with stage 4–5 chronic kidney disease not yet on dialysis
- Comparative Efficacy and Safety of Febuxostat and Allopurinol in the Treatment of Hyperuricemia: A Bayesian Network Meta-analysis
- What is the Best Choice for Urate-lowering Therapy for Korean?
- The Efficacy and Safety of Febuxostat in Korean Patients with Gout
- Updates in the management of gouty arthritis