Korean J Urol.
2006 Jun;47(6):578-585. 10.4111/kju.2006.47.6.578.
The Anatomic Distribution and Pathological Characteristics of Prostate Cancer: A Mapping Analysis
- Affiliations
-
- 1Department of Urology and Biochemical Engineering, University of Ulsan College of Medicine, Seoul, Korea. hjahn@amc.seoul.kr
- KMID: 2294229
- DOI: http://doi.org/10.4111/kju.2006.47.6.578
Abstract
-
PURPOSE: We mapped the location of prostate cancer in Korean men, and investigated the volume and tumor distribution in relation to clinicopathological variables.
MATERIALS AND METHODS
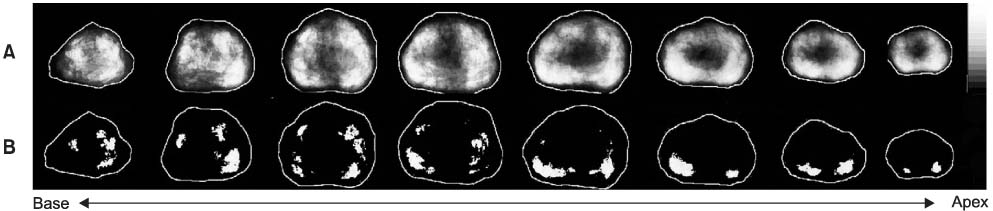
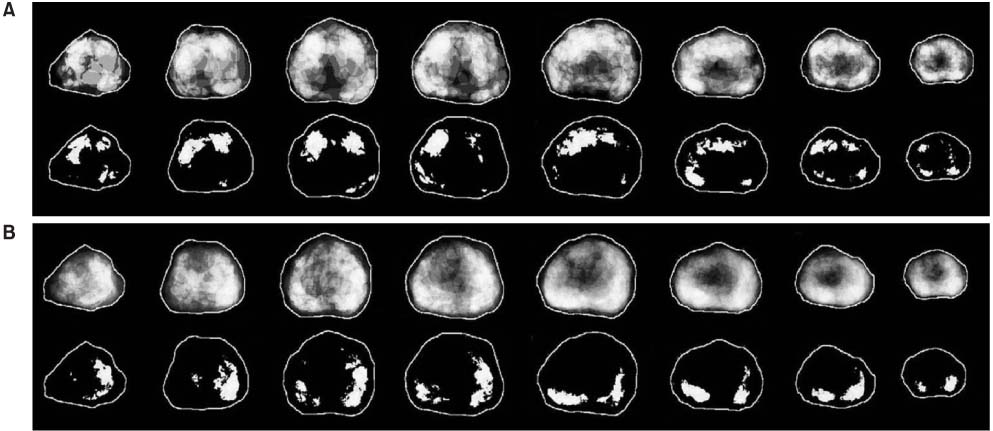
The volume of cancer and the anatomic location of each tumor foci were determined from 186 radical prostatectomy specimens, which were digitized to fit into a prototype prostate model. Using the computer-based digital images, the zonal cancer volume and distributional frequency were analyzed with respect to the clinical and pathological parameters, which were demonstrated in gray scales.
RESULTS
The preoperative serum prostate-specific antigen (PSA) level ranged from 2.0 to 38.9ng/ml. The mean cancer volume of the 186 specimens was 4.5ml (median 1.9ml, range 0.01-37.7). The impalpable cancers were located more anteriorly and in the transition zone, and were also were smaller in volume (2.7ml vs. 5.5ml, p=0.004) than the palpable cancers. Cancers with seminal vesicle invasion were located more medially in the peripheral zone, and were larger in volume than organ-confined cancers or cancers with extracapsular extension (13.2ml vs. 3.0ml, p<0.001). For Gleason scores of 2-6, 7, and 8-10, the mean cancer volumes were 2.2, 3.7 and 8.2ml, respectively (p<0.001). High grade cancers were located more medially in the peripheral zone, especially when approaching the apex.
CONCLUSIONS
T1c cancers are located more anteriorly and in the transition zone; therefore, inclusion of these areas for targeted biopsy may help to improve the detection of cancer in patients with elevated PSA levels and impalpable prostate cancer. A medial location of seminal vesicle invasive cancers may imply an ejaculatory ducts route of invasion rather than a direct extracapsular extension.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Statistical 3D Distribution Analysis of Prostate Cancers in Korean Using Digital Processing Techniques
Pil June Pak, Dong Ik Shin, Young Mi Cho, Se Kyeong Joo, Soo Jin Huh
Healthc Inform Res. 2011;17(1):51-57. doi: 10.4258/hir.2011.17.1.51.
Reference
-
1. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA cancer. J Clin. 2000. 50:7–33.2. 2001 Annual report of the Korea central cancer registry (based on registered data from 134 hospitals). 2003. Korea central cancer registry, ministry of health and welfare republic of Korea.3. Hodge KK, McNeal JE, Stamey TA. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989. 142:66–70.4. McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988. 12:897–906.5. Chen ME, Johnston DA, Tang K, Babaian RJ, Troncoso P. Detailed mapping of prostate carcinoma foci: biopsy strategy implications. Cancer. 2000. 89:1800–1809.6. Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of non-palpable (stage T1c) prostate cancer. JAMA. 1994. 271:368–374.7. Igel TC, Knight MK, Young PR, Wehle MJ, Petrou SP, Broderick GA, et al. Systematic transperineal ultrasound guided template biopsy of the prostate in patients at high risk. J Urol. 2001. 165:1575–1579.8. Demura T, Hioka T, Furuno T, Kaneta T, Gotoda H, Muraoka S, et al. Differences in tumor core distribution between palpable and nonpalpable prostate tumors in patients diagnosed using extensive transperineal ultrasound-guided template prostate biopsy. Cancer. 2005. 103:1826–1832.9. Noguchi M, Stamey TA, McNeal JE, Yemoto CE. An analysis of 148 consecutive transition zone cancers: clinical and histological characteristics. J Urol. 2000. 163:1751–1755.10. McNeal JE, Bostwick DG. Bostwick DG, editor. Anatomy of the prostate: implication for disease. Pathology of the prostate. 1990. 1st ed. New York: Churchill-Livingstone;1–14.11. Stamey TA, Dietrick DD, Issa MM. Large, organ confined, impalpable transition zone prostate cancer: association with metastatic level of prostate specific antigen. J Urol. 1993. 149:510–515.12. Keetch DW, Catalona WJ. Prostatic transition zone biopsies in men with previous negative biopsies and persistently elevated serum prostate specific antigen values. J Urol. 1995. 154:1795–1797.13. McNeal J, Noldus J. Limitations of transition zone needle biopsy findings in the prediction of transition zone cancer and tissue composition of benign nodular hyperplasia. Urology. 1996. 48:751–756.14. Chen ME, Troncoso P, Tang K, Babaian RJ, Johnston D. Comparison of prostate biopsy schemes by computer simulation. Urology. 1999. 53:951–960.15. Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1000 consecutive patients. J Urol. 2002. 167:528–534.16. Villers AA, McNeal JE, Redwine EA, Freiha FS, Stamey TA. Pathogenesis and biological significance of seminal vesicle invasion in prostatic adenocarcinoma. J Urol. 1990. 143:1183–1187.17. Ohori M, Scardino PT, Lapin SL, Seale-Hawkins C, Link J, Wheeler TM. The mechanisms and prognostic significance of seminal vesicle involvement by prostate cancer. Am J Surg Pathol. 1993. 17:1252–1261.18. Lee F, Torp-Pedersen ST, Siders DB, Littrup PJ, McLeary RD. Transrectal ultrasound in the diagnosis and staging of prostatic carcinoma. Radiology. 1989. 170:609–615.19. Ayala AG, Ro JY, Babaian R, Troncoso P, Grignon DJ. The prostatic capsule: Does it exist? Its importance in the staging and treatment of prostatic carcinoma. Am J Surg Pathol. 1989. 13:21–27.20. Lee TK, Chung TG, Kim CS. Age-specific reference ranges for prostate specific antigen from a health center in Korea. Korean J Urol. 1999. 40:583–588.21. Lee SE, Kim DY, Kwak C. Interrelationship among age, prostate specific antigen and prostate volume in Korean men living at the Metropolitan area. Korean J Urol. 1999. 40:1311–1317.22. Cho HS, Kim CS, Ahn H. The pathological characteristics of localized prostate cancer, nanaged with radical prostatectomy. Korean J Urol. 2002. 43:938–943.23. Furuya Y, Ohta S, Sato N, Kotake T, Masai M. Comparison of T1c versus T2 prostate cancers in Japanese patients undergoing radical prostatectomy. Int Urol Nephrol. 2002. 33:73–76.24. Song CR, Kim JB, Chung H, Kim CS, Ro JY, Ahn HJ. Nomograms for the prediction of the pathological stage of the clinically localized prostate cancer in Korean men. Korean J Urol. 2003. 44:753–758.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathologic Features of Korean Prostate Adenocarcinoma: Mapping Analysis of 83 Cases
- A study on double contrast study in early gastric cancer
- The Pathological Characteristics of Localized Prostate Cancer, Managed with Radical Prostatectomy
- Statistical 3D Distribution Analysis of Prostate Cancers in Korean Using Digital Processing Techniques
- Racial Differences in Prostate Cancer Characteristics and Cancer-Specific Mortality: An Overview