Korean J Urol.
2006 Feb;47(2):143-149. 10.4111/kju.2006.47.2.143.
Anticancer Efficacy and Toxicity of Oral GMO-paclitaxel in a Hormone Refractory Prostate Cancer Model
- Affiliations
-
- 1Department of Urology, College of Medicine, The Catholic University of Korea. ksw1227@catholic.ac.kr
- 2Korean Institute of Science and Technology, Korea.
- 3College of Pharmacy, Chung-Ang University, Seoul, Korea.
- KMID: 2294192
- DOI: http://doi.org/10.4111/kju.2006.47.2.143
Abstract
-
PURPOSE: We wanted to evaluate the efficacy and toxicity of the newly developed oral glyceryl monooleate (GMO)-paclitaxel in a hormone refractory prostate cancer model.
MATERIALS AND METHODS
A paclitaxel formulation was prepared from GMO, tricaprylin, Tween(R) 80 and paclitaxel. The tumor cells of prostate cancer (DU-145 cells) were incubated and then put into different paclitaxel concentrations. The tumoricidal activity was measured by using an indirect methylthiazol-2-yl-2,5-diphenyl tetrazolium bromide (MTT) assay. Cells of the DU-145 cell line were subcutaneously heterotransplanted into 18 nude mice, and they developed prostate cancer. The 18 mice were divided into 3 groups; the control group was injected with the DU-145 cell line (n=6), the GMO group was injected with GMO after the DU-145 cells were injected (n=6), and the oral GMO-paclitaxel group was injected with oral GMO-paclitaxel after the DU-145 cells were injected (n=6). The tumor volume was measured every week and the main organs were evaluated pathologically to determine the toxicity.
RESULTS
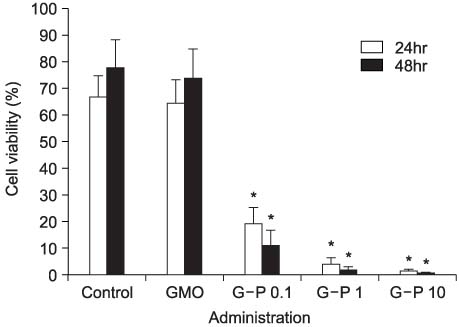
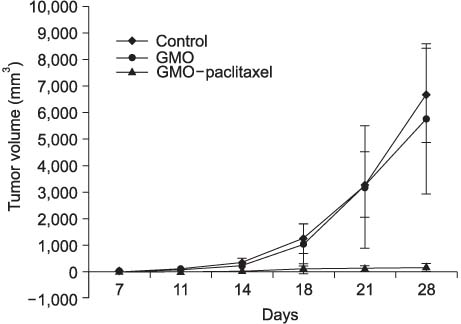
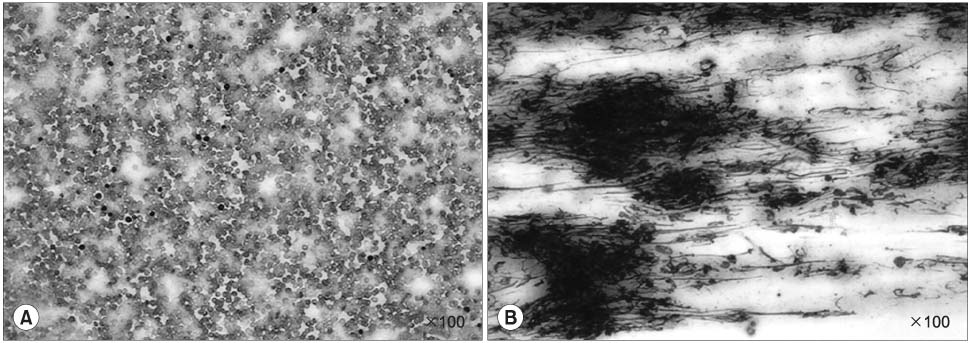
On the MTT assay, the control group and the GMO group did not display cytotoxicity. However, treatment with the various GMO-paclitaxel formulations (0.1 microgram/ml, 1 microgram/ml, 10 microgram/ml) for treating the DU-145 cell line cancer induced cytotoxicity in a dose dependent fashion. The tumor volumes were not significantly changed in the group that was administered oral GMO-paclitaxel. However, there were significantly increased tumor volumes in the control group and the GMO group (p<0.05). Toxic changes were not detected in liver and kidney, and there was normal cellularity with a normal myeloid:erythroid ratio in the mice after the administration of oral GMO-paclitaxel.
CONCLUSIONS
The newly developed oral GMO-paclitaxel has a remarkable cytotoxic effect against DU-145 cells without systemic toxicity. Therefore, oral GMO-paclitaxel therapy promises to be a safe and effective modality for treating hormone refractory prostate cancer, and it can possibly replace IV paclitaxel.
MeSH Terms
Figure
Reference
-
1. National Cancer Center. http://www.ncc.re.kr.2. Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997. 47:5–27.3. Muthuramalingam SR, Patel K, Protheroe A. Management of patients with hormone refractory prostate cancer. Clin Oncol. 2004. 16:505–516.4. Kantoff PW. New agents in the therapy of hormone-refractory patients with prostate cancer. Semin Oncol. 1995. 22:1 Suppl 1. 32–34.5. Oliverio G, Canuti D, Tononi A. Paclitaxel efficacy and tolerability in second- line treatment of refractory and relapsed ovarian cancer patients. J Chemother. 1999. 11:301–305.6. Orr C. Becher P, editor. Emulsion droplet size data. Encyclopedia of emulsion technology. 1983. 1st ed. New York: Marcel Dekker;369–404.7. Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966. 50:219–244.8. Leung SY, Jackson J, Miyake H, Burt H, Gleave ME. Polymeric micellar paclitaxel phosphorylates Bcl-2 and induces apoptotic regression of androgen-independent LNCaP prostate tumors. Prostate. 2000. 44:156–163.9. Athanasiadis A, Tsavdaridis D, Rigatos SK, Athanasiadis I, Pergantas N, Stathopoulos GP. Hormone refractory advanced prostate cancer treated with estramustine and paclitaxel combination. Anticancer Res. 2003. 23:3085–3088.10. Smith DC, Chay CH, Dunn RL, Fardig J, Esper P, Olson K, et al. Phase II trial of paclitaxel, estramustine, etoposide, and carboplatin in the treatment of patients with hormone-refractory prostate carcinoma. Cancer. 2003. 98:269–276.11. Vaughn DJ, Brown AW Jr, Harker WG, Huh S, Miller L, Rinaldi D, et al. Multicenter Phase II study of estramustine phosphate plus weekly paclitaxel in patients with androgen-independent prostate carcinoma. Cancer. 2004. 100:746–750.12. Stein CA. Mechanisms of action of taxanes in prostate cancer. Semin Oncol. 1999. 26:5 Suppl 17. 3–7.13. Haris AW, Lowenthal JW. Cells of some cultured lymphoma lines are killed rapidly by x-rays and bleomycin. Int J Radiat Biol Relat Stud Phys Chem Med. 1982. 42:111–116.14. Manfredi JJ, Parness J, Horwitz SB. Taxol binds to cellular microtubules. J Cell Biol. 1982. 94:688–696.15. Rowinsky EK, Donehwer RC, Jones RJ, Tucker RW. Microtubule changes and cytotoxicity in leukemic cell line treated with taxol. Cancer Res. 1988. 48:4093–4100.16. Rowinsky EK, Onetto N, Canetta RM, Arbuck SG. Taxol: the first of the taxanes, an important new class of antitumor agents. Semin Oncol. 1992. 19:646–662.17. Jackson JK, Gleave ME, Yago V, Beraldi E, Hunter WL, Burt HM. The suppression of human prostate tumor growth in mice by the intratumoral injection of a slow-release polymeric paste formulation of paclitaxel. Cancer Res. 2000. 60:4146–4151.18. Oudard S, Legrier ME, Boye K, Brass-Goncalves R, Pinieux G, De Cremoux P, et al. Activity of docetaxel with or without estramustine phosphate versus mitoxantrone in androgen dependent and independent human prostate cancer xenografts. J Urol. 2003. 169:1729–1734.19. Canil CM, Tannock IF. Is there a role for chemotherapy in prostate cancer? Br J Cancer. 2004. 91:1005–1011.20. Malingre MM, Terwogt JM, Beijnen JH, Rosing H, Koopman FJ, van Tellingen O, et al. Phase I and pharmacokinetic study of oral paclitaxel. J Clin Oncol. 2000. 18:2468–2475.21. Feng SS, Mu L, Win KY, Huang G. Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr Med Chem. 2004. 11:413–424.22. Rose WC, Clark JL, Lee FY, Casazza AM. Preclinical antitumor activity of water-soluble paclitaxel derivatives. Cancer Chemother Pharmacol. 1997. 39:486–492.23. Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990. 8:1263–1268.24. Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH. Nonlinear pharmacokinetics of paclitaxel in mice results from the pharmaceutical vehicle Cremophor EL. Cancer Res. 1996. 56:2112–2115.25. Kimura Y, Aoki J, Kohno M, Ooka H, Tsuruo T, Nakanishi O. P-glycoprotein inhibition by the multidrug resistance-reversing agent MS-209 enhances bioavailability and antitumor efficacy of orally administered paclitaxel. Cancer Chemother Pharmacol. 2002. 49:322–328.26. Nielsen LS, Schubert L, Hansen J. Bioadhesive drug delivery systems. I. Characterisation of mucoadhesive properties of systems based on glyceryl monooleate and glyceryl monolinoleate. Eur J Pharm Sci. 1998. 6:231–239.27. Engstrom S, Engstrom L. Phase behavior of the lidocaine-monoolein-water system. Int J Pharm. 1992. 79:113–122.28. Sallam AS, Khalil E, Ibrahim H, Freij I. Formulation of an oral dosage form utilizing the properties of cubic liquid crystalline phases of glyceryl monooleate. Eur J Pharm Biopharm. 2002. 53:343–352.29. van Asperen J, van Tellingen O, van der Valk MA, Rozenhart M, Beijnen JH. Enhanced oral absorption and decreased elimination of paclitaxel in mice cotreated with cyclosporin A. Clin Cancer Res. 1998. 4:2293–2297.30. Rose WC, Lee FY, Golik J, Kadow J. Preclinical oral antitumor activity of BMS-185660, a paclitaxel derivative. Cancer Chemother Pharmacol. 2000. 46:246–250.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anticancer Activity of Intravesical Glyceryl Monooleate (GMO)-Paclitaxel Therapy in Murine Superficial Transitional Cell Carcinoma Model Induced by BBN
- Efficacy of Paclitaxel-Loaded Bioadhesive Drug Deliver System Based on Glyceryl Monooleate Nanoparticle in an Orthotopic Murine Bladder Cancer Model
- Bioadhesive Drug Delivery System for the Intravesical Administration of Paclitaxel in Rabbits
- The Anticancer Efficacy and Toxicity of Oral Paclitaxel- Loaded Lipid Nanoparticle in a C3H2 Bladder Cancer Mice
- Phase I Clinical Trial of Paclitaxel Plus Ifosfamide for the Patients with Refractory Ovarian Cancer