J Breast Cancer.
2010 Mar;13(1):115-119. 10.4048/jbc.2010.13.1.115.
Primary Neuroendocrine Carcinoma in the Breast: A Case Report
- Affiliations
-
- 1Department of General Surgery, Gazi University School of Medicine, Ankara, Turkey. bsalman@gazi.edu.tr
- KMID: 2286569
- DOI: http://doi.org/10.4048/jbc.2010.13.1.115
Abstract
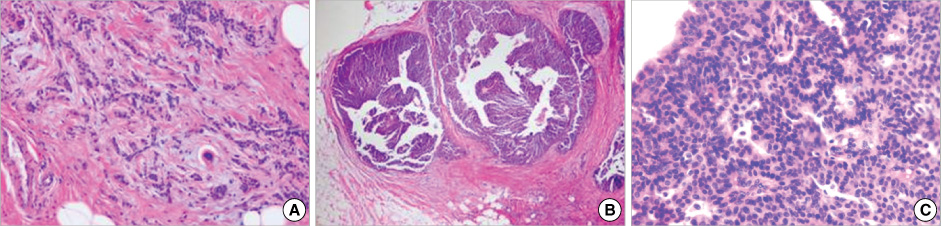
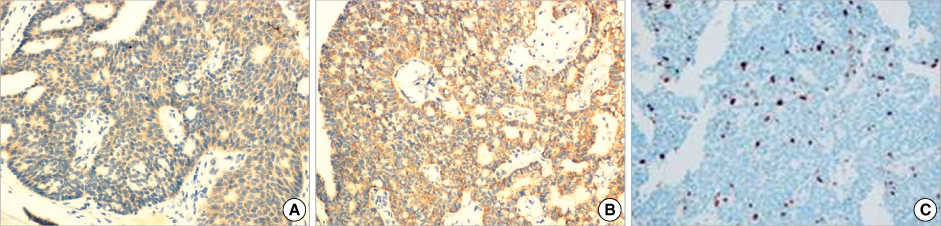
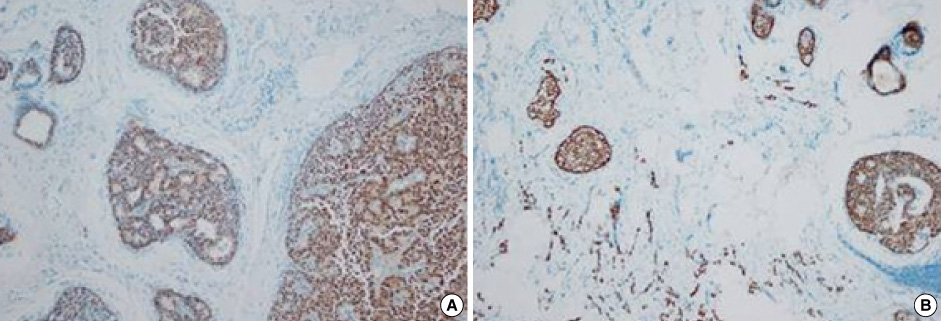
- Primary neuroendocrine carcinoma of the breast is an extremely rare neoplasm that differs from other types of breast carcinoma according to the pathological features. We describe here a case of primary neuroendocrine carcinoma in a 72-year-old woman who complainted of a lump in her left breast. After examination and evaluation, the diagnosis was made by a core needle biopsy and the subsequent immunohistochemical examination of the specimen revealed more detailed information. We performed modified radical mastectomy, which showed no axillary node involvement. The patient was given neither radiation nor chemotherapy, however, but hormonotherapy was started postoperatively. Any recurrence has not been demonstrated at 12 months after the operation.
Keyword
MeSH Terms
Figure
Reference
-
1. Upalakalin JN, Collins LC, Tawa N, Parangi S. Carcinoid tumors in the breast. Am J Surg. 2006. 191:799–805.
Article2. Sapino A, Righi L, Cassoni P, Papotti M, Pietribiasi F, Bussolati G. Expression of the neuroendocrine phenotype in carcinomas of the breast. Semin Diagn Pathol. 2000. 17:127–137.3. Berruti A, Saini A, Leonardo E, Cappia S, Borasio P, Dogliotti L. Management of neuroendocrine differentiated breast carcinoma. Breast. 2004. 13:527–529.
Article4. Papotti M, Macrì L, Finzi G, Capella C, Eusebi V, Bussolati G. Neuroendocrine differentiation in carcinoma of the breast: a study of 51 cases. Semin Diagn Pathol. 1989. 6:174–188.5. Tavassoli FA, Devilee P. World Health Organization Classification of Tumors. Pathology and genetics of tumors of the breast and female genital organs. 2003. Lyon: International Agency for Research on Cancer (IARC) Press;32–34.6. Scopsi L, Andreola S, Pilotti S, Testori A, Baldini MT, Leoni F, et al. Argyrophilia and granin (chromogranin/secrotogranin) expression in female breast carcinomas. Am J Surg Pathol. 1992. 16:561–576.
Article7. Zekioğlu O, Erhan Y, Ciris M, Bayramoğlu H. Neuroendocrine differentiated breast carcinoma of the breast: a distinct entity. Breast. 2003. 12:251–257.8. Günhan-Bilgen I, Zekioglu O, Ustün EE, Memis A, Erhan Y. Neuroendocrine differentiated breast carcinoma: imaging features correlated with clinical and histopathological findings. Eur Radiol. 2003. 13:788–793.
Article9. Fujimoto Y, Yagyu R, Murase K, Kawajiri H, Ohtani H, Arimoto Y, et al. A case of solid neuroendocrine carcinoma of the breast in a 40-year-old woman. Breast Cancer. 2007. 14:250–253.
Article10. Wade PM Jr, Mills SE, Read M, Cloud W, Lambert MJ 3rd, Smith RE. Small cell neuroendocrine (oat cell) carcinoma of the breast. Cancer. 1983. 52:121–125.
Article11. Tsang WY, Chan KC. Endocrine ductal carcinoma in situ (E-DCIS) of the breast: a form of low-grade DCIS with distinctive clinicapathologic and biologic characteristics. Am J Surg Pathol. 1996. 20:921–943.12. Maluf HM, Koerner FC. Carcinoma of the breast with NE differentiation. A review. Virchow Arch. 1994. 425:449–457.13. Sapino A, Bussolati G. Is detection of endocrine cells in breast adenocarcinoma of diagnostic and clinical significance? Histopathology. 2002. 40:211–214.
Article14. Mirza IA, Hahab N. Small cell carcinoma of the breast. Semin Oncol. 2007. 34:64–66.
Article15. Shin SS, DeLellis RA, Ying L, Rosen PP. Small cell carcinoma of the breast. A clinical pathologic and immunohistochemical study of nine patients. Am J Surg Pathol. 2000. 24:1231–1238.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary Neuroendocrine Carcinoma of the Breast: A Case Report and Literature Review
- Small-cell neuroendocrine carcinoma of the breast
- Mammographic, Sonographic, and MRI Features of Primary Neuroendocrine Carcinoma of the Breast: A Case Report
- Primary Breast Carcinoma with Neuroendocrine Features: Imaging Features on Mammography and Ultrasonography
- Primary Small Cell Neuroendocrine Carcinoma of the Breast: A Case Report With Literature Review