Korean J Physiol Pharmacol.
2015 Mar;19(2):83-88. 10.4196/kjpp.2015.19.2.83.
Dynamic Frequency of Blood CD4+CD25+ Regulatory T Cells in Rats with Collagen-induced Arthritis
- Affiliations
-
- 1Department of Pharmacy, Shanghai Ninth People's Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200011, China.
- 2School of Pharmacy, Anhui Medical University, Hefei 230032, Anhui, China. aydlijun@sohu.com
- 3Bone Research Program, ANZAC Research Institute, University of Sydney, NSW 2139, Sydney, Australia.
- KMID: 2285558
- DOI: http://doi.org/10.4196/kjpp.2015.19.2.83
Abstract
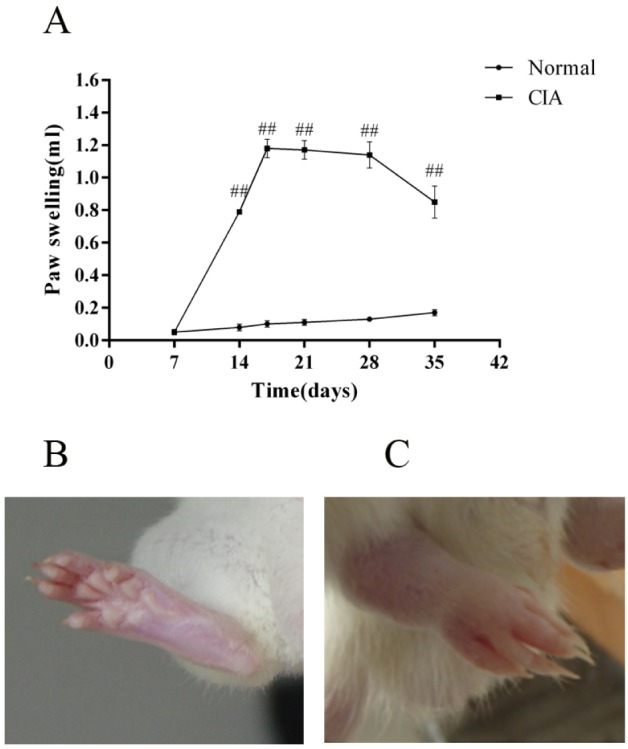
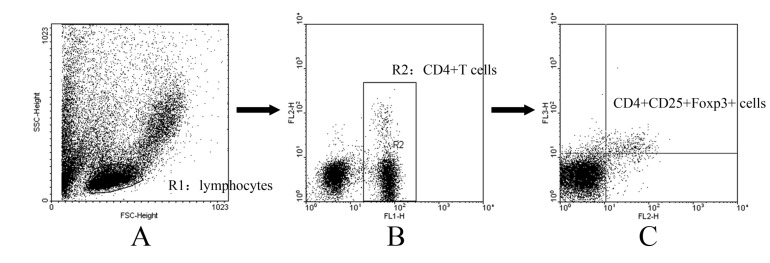
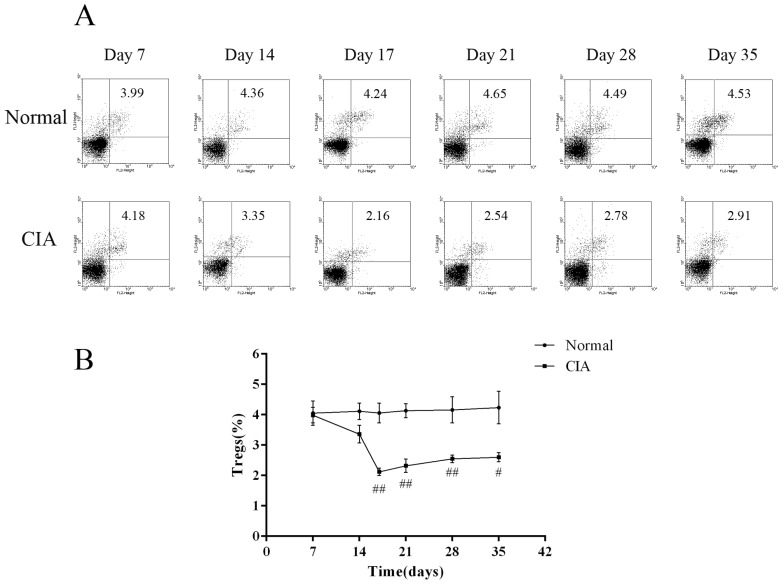
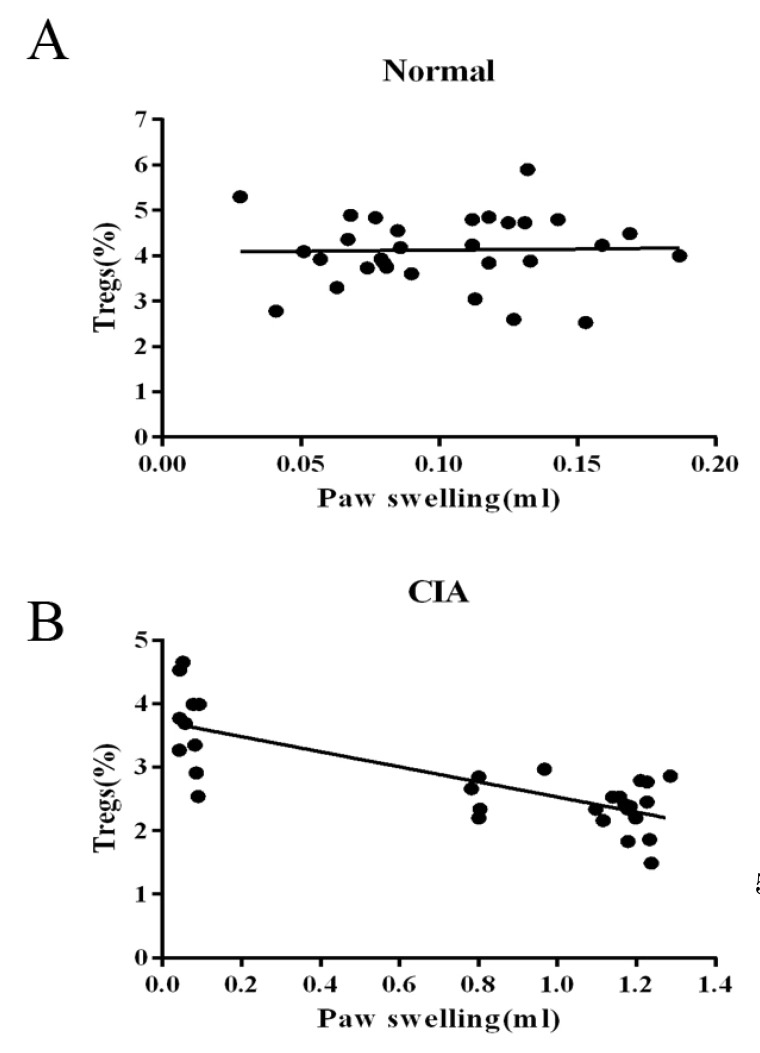
- CD4+CD25+ regulatory T cells (CD4+CD25+ Tregs) have been shown to play a regulatory or suppressive role in the immune response and are possibly relevant to the pathogenesis of autoimmune diseases. In the present study, we attempted to investigate the frequency of CD4+CD25+ Tregs in peripheral blood (PB) of collagen-induced arthritis (CIA) rats during the development of arthritis, to determine whether their frequency is involved in the immunoregulation of this disease. The results showed that normal rats had similar frequencies of CD4+CD25+ Tregs in PB during the experiment time, expressed as a percentage of CD4+CD25+Foxp3+ T cells among the CD4+ T lymphocyte population. In contrast, the frequency of CD4+CD25+Foxp3+ T cells in CIA rats was found to change during the development of arthritis. In CIA rats, there is a significant negative correlation between the frequency of CD4+CD25+Foxp3+ T cells and paw swelling (r=-0.786, p< 0.01). The relationship between the frequency of CD4+CD25+Foxp3+ T and immune activation was not found in normal rats. During the time course, the frequency of CD4+CD25+Foxp3+ T was lower in CIA rats than in normal ones. The data suggest that the frequency of PB CD4+CD25+ Tregs may be a promising marker for arthritis activity.
Keyword
MeSH Terms
Figure
Reference
-
1. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995; 155:1151–1164. PMID: 7636184.2. Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, Procaccini C, La Cava A, Matarese G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014; 20:69–74. PMID: 24317118.
Article3. Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007; 7:305–310. PMID: 17380159.
Article4. Mu J, Tai X, Iyer SS, Weissman JD, Singer A, Singer DS. Regulation of MHC class I expression by Foxp3 and its effect on regulatory T cell function. J Immunol. 2014; 192:2892–2903. PMID: 24523508.
Article5. Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003; 48:1452–1460. PMID: 12746920.
Article6. Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005; 52:2212–2221. PMID: 15986351.
Article7. Frey O, Petrow PK, Gajda M, Siegmund K, Huehn J, Scheffold A, Hamann A, Radbruch A, Bräuer R. The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+CD25+ T cells. Arthritis Res Ther. 2005; 7:R291–R301. PMID: 15743476.8. Cao D, Malmström V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003; 33:215–223. PMID: 12594850.
Article9. Möttönen M, Heikkinen J, Mustonen L, Isomäki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005; 140:360–367. PMID: 15807863.10. Liu MF, Wang CR, Fung LL, Lin LH, Tsai CN. The presence of cytokine-suppressive CD4+CD25+ T cells in the peripheral blood and synovial fluid of patients with rheumatoid arthritis. Scand J Immunol. 2005; 62:312–317. PMID: 16179019.
Article11. van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004; 50:2775–2785. PMID: 15457445.
Article12. Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmström V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004; 6:R335–R346. PMID: 15225369.13. Lawson CA, Brown AK, Bejarano V, Douglas SH, Burgoyne CH, Greenstein AS, Boylston AW, Emery P, Ponchel F, Isaacs JD. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford). 2006; 45:1210–1217. PMID: 16571607.
Article14. Kong W, Li J, Wang TY, Huang LY. Establishment and evaluation of collagen-induced arthritis model in rats. Acta Univ Med Anhui. 2008; 43:3–7.15. Wang TY, Li J, Jin Z, Wu F, Zhou Q. Inhibitory effect of TGF-β1 on NO production in peritoneal macrophages from collagen-induced arthritis rats involving the LPS-TLR4 pathway. Mol Med Rep. 2013; 8:1143–1148. PMID: 23970162.
Article16. Vierboom MP, Jonker M, Bontrop RE, 't Hart B. Modeling human arthritic diseases in nonhuman primates. Arthritis Res Ther. 2005; 7:145–154. PMID: 15987497.17. Kim YO, Hong SJ, Yim SV. The efficacy of shikonin on cartilage protection in a mouse model of rheumatoid arthritis. Korean J Physiol Pharmacol. 2010; 14:199–204. PMID: 20827333.
Article18. Langier S, Sade K, Kivity S. Regulatory T cells: the suppressor arm of the immune system. Autoimmun Rev. 2010; 10:112–115. PMID: 20807589.
Article19. Anderson AE, Isaacs JD. Tregs and rheumatoid arthritis. Acta Reumatol Port. 2008; 33:17–33. PMID: 18344919.20. Nolte-'t Hoen EN, Boot EP, Wagenaar-Hilbers JP, van Bilsen JH, Arkesteijn GJ, Storm G, Everse LA, van Eden W, Wauben MH. Identification and monitoring of effector and regulatory T cells during experimental arthritis based on differential expression of CD25 and CD134. J Leukoc Biol. 2008; 83:112–121. PMID: 17928458.21. Kim JM, Joo HG. Immunostimulatory Effects of β-glucan Purified from Paenibacillus polymyxa JB115 on Mouse Splenocytes. Korean J Physiol Pharmacol. 2012; 16:225–230. PMID: 22915986.22. Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014; 15:580–587. PMID: 24728351.
Article23. Wang TY, Li J, Li CY, Jin Y, Lü XW, Wang XH, Zhou Q. Leflunomide induces immunosuppression in collagen-induced arthritis rats by upregulating CD4+CD25+ regulatory T cells. Can J Physiol Pharmacol. 2010; 88:45–53. PMID: 20130738.24. Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Vasoactive intestinal peptide induces CD4+,CD25+ T regulatory cells with therapeutic effect in collagen-induced arthritis. Arthritis Rheum. 2006; 54:864–876. PMID: 16508968.
Article25. Auci D, Kaler L, Subramanian S, Huang Y, Frincke J, Reading C, Offner H. A new orally bioavailable synthetic androstene inhibits collagen-induced arthritis in the mouse: androstene hormones as regulators of regulatory T cells. Ann N Y Acad Sci. 2007; 1110:630–640. PMID: 17911478.
Article26. Gonzalez-Rey E, Chorny A, O'Valle F, Delgado M. Adrenomedullin protects from experimental arthritis by down-regulating inflammation and Th1 response and inducing regulatory T cells. Am J Pathol. 2007; 170:263–271. PMID: 17200199.
Article27. Su D, Shen M, Gu B, Wang X, Wang D, Li X, Sun L. 99 Tc-methylene diphosphonate improves rheumatoid arthritis disease activity by increasing the frequency of peripheral γδ T cells and CD4+ CD25+ Foxp3+ Tregs. Int J Rheum Dis. 2014; doi: 10.1111/1756-185X.12292. [Epub ahead of print].
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- IL-4 Induces CD4+CD25+ Regulatory T Cells from CD4+CD25- T Cells in Peripheral Blood
- A Study on the Number of Circulating CD4+CD25+Foxp3+ Regulatory T Cells and CD4+CD25-Foxp3+ T Cells in Psoriasis
- Suppression of Pathogenic Autoreactive CD4+ T Cells by CD137-mediated Expansion of CD4+CD25+ Regulatory T Cells in Graves' Disease
- Anti-CD3 Antibody Induces IL-10-producing CD4+CD25+ Regulatory T Cells, Which Suppress T Cell Response in Rheumatoid Arthritis Patients