Korean J Physiol Pharmacol.
2014 Dec;18(6):503-508. 10.4196/kjpp.2014.18.6.503.
Transient Receptor Potential C4/5 Like Channel Is Involved in Stretch-Induced Spontaneous Uterine Contraction of Pregnant Rat
- Affiliations
-
- 1Department of Physiology, Yonsei University College of Medicine, Seoul 120-752, Korea. dsahn@yuhs.ac
- KMID: 2285553
- DOI: http://doi.org/10.4196/kjpp.2014.18.6.503
Abstract
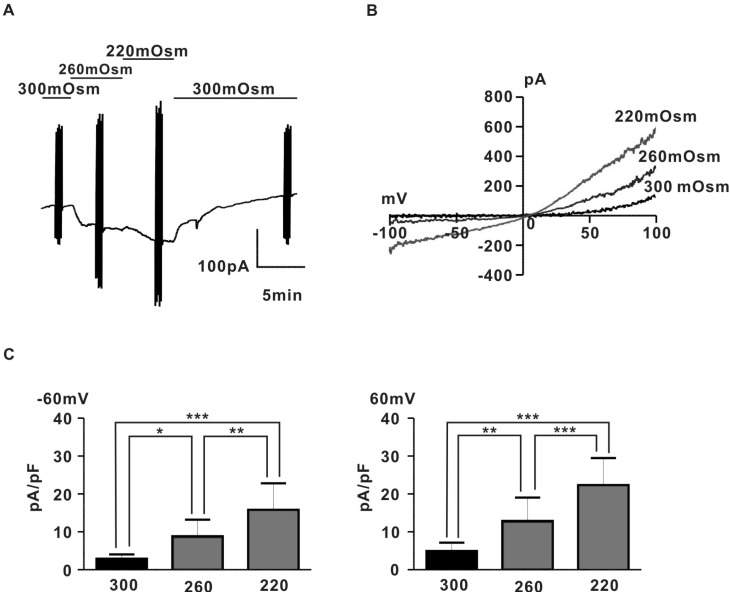
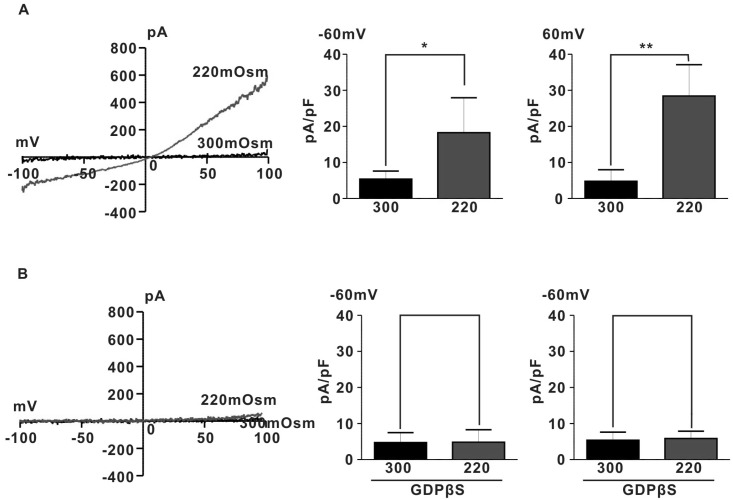
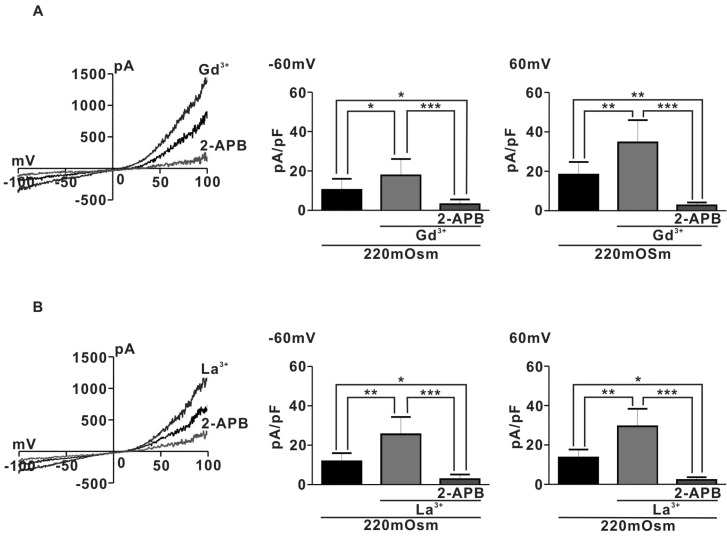
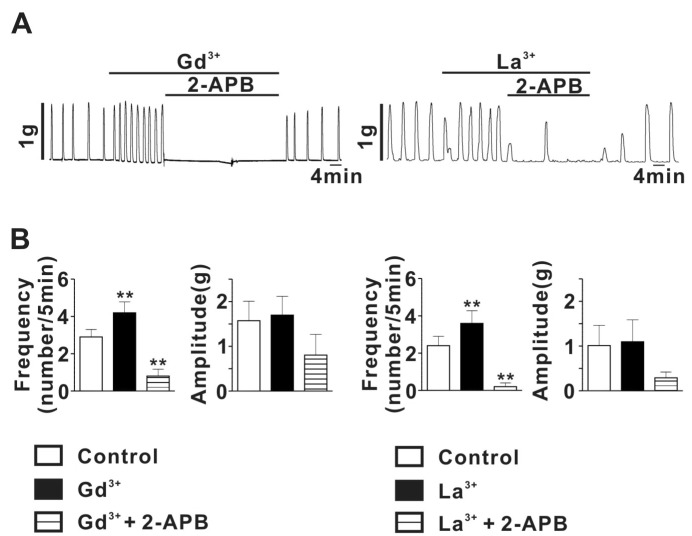
- Spontaneous myometrial contraction (SMC) in pregnant uterus is greatly related with gestational age and growing in frequency and amplitude toward the end of gestation to initiate labor. But, an accurate mechanism has not been elucidated. In human and rat uterus, all TRPCs except TRPC2 are expressed in pregnant myometrium and among them, TRPC4 are predominant throughout gestation, suggesting a possible role in regulation of SMC. Therefore, we investigated whether the TRP channel may be involved SMC evoked by mechanical stretch in pregnant myometrial strips of rat using isometric tension measurement and patch-clamp technique. In the present results, hypoosmotic cell swelling activated a potent outward rectifying current in G protein-dependent manner in rat pregnant myocyte. The current was significantly potentiated by 1microM lanthanides (a potent TRPC4/5 stimulator) and suppressed by 10microM 2-APB (TRPC4-7 inhibitor). In addition, in isometric tension experiment, SMC which was evoked by passive stretch was greatly potentiated by lanthanide (1microM) and suppressed by 2-APB (10microM), suggesting a possible involvement of TRPC4/5 channel in regulation of SMC in pregnant myometrium. These results provide a possible cellular mechanism for regulation of SMC during pregnancy and provide basic information for developing a new agent for treatment of premature labor.
MeSH Terms
Figure
Cited by 1 articles
-
Identification of Lys49-PLA2 from crude venom of
Crotalus atrox as a human neutrophil-calcium modulating protein
Md. Tipu Sultan, Hong-Mei Li, Yong Zu Lee, Soon Sung Lim, Dong-Keun Song
Korean J Physiol Pharmacol. 2016;20(2):177-183. doi: 10.4196/kjpp.2016.20.2.177.
Reference
-
1. Bengtsson B. Factors of importance for regulation of uterine contractile activity. Acta Obstet Gynecol Scand Suppl. 1982; 108:13–16. PMID: 6957124.
Article2. Olson DM, Mijovic JE, Sadowsky DW. Control of human parturition. Semin Perinatol. 1995; 19:52–63. PMID: 7754411.
Article3. Wray S. The role of mechanical and hormonal stimuli on uterine involution in the rat. J Physiol. 1982; 328:1–9. PMID: 7131308.
Article4. Wray S. Uterine contraction and physiological mechanisms of modulation. Am J Physiol. 1993; 264:C1–C18. PMID: 8430759.
Article5. Kasai Y, Tsutsumi O, Taketani Y, Endo M, Iino M. Stretch-induced enhancement of contractions in uterine smooth muscle of rats. J Physiol. 1995; 486:373–384. PMID: 7473204.
Article6. Moutquin JM. Classification and heterogeneity of preterm birth. BJOG. 2003; 110(Suppl 20):30–33. PMID: 12763108.
Article8. Kleinhaus AL, Kao CY. Electrophysiological actions of oxytocin on the rabbit myometrium. J Gen Physiol. 1969; 53:758–780. PMID: 5783010.
Article9. Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003; 93:829–838. PMID: 14512441.
Article10. Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol. 2004; 559:685–706. PMID: 15272031.11. Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatinrelated cation channel TRPM3. J Biol Chem. 2003; 278:21493–21501. PMID: 12672799.
Article12. Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004; 95:922–929. PMID: 15472118.
Article13. Kraft R, Harteneck C. The mammalian melastatin-related transient receptor potential cation channels: an overview. Pflugers Arch. 2005; 451:204–211. PMID: 15895246.
Article14. Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther. 2006; 112:744–760. PMID: 16842858.
Article15. Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006; 99:119–131. PMID: 16857972.
Article16. Dalrymple A, Slater DM, Beech D, Poston L, Tribe RM. Molecular identification and localization of Trp homologues, putative calcium channels, in pregnant human uterus. Mol Hum Reprod. 2002; 8:946–951. PMID: 12356946.
Article17. Babich LG, Ku CY, Young HW, Huang H, Blackburn MR, Sanborn BM. Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol Reprod. 2004; 70:919–924. PMID: 14627551.18. Ku CY, Babich L, Word RA, Zhong M, Ulloa A, Monga M, Sanborn BM. Expression of transient receptor channel proteins in human fundal myometrium in pregnancy. J Soc Gynecol Investig. 2006; 13:217–225.
Article19. Asano M, Nomura Y. Comparison of inhibitory effects of Y-27632, a Rho kinase inhibitor, in strips of small and large mesenteric arteries from spontaneously hypertensive and normotensive Wistar-Kyoto rats. Hypertens Res. 2003; 26:97–106. PMID: 12661918.
Article20. Holz GG 4th, Rane SG, Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986; 319:670–672. PMID: 2419757.
Article21. Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007; 282:33868–33878. PMID: 17884814.
Article22. Kuriyama H, Suzuki H. Changes in electrical properties of rat myometrium during gestation and following hormonal treatments. J Physiol. 1976; 260:315–333. PMID: 978524.
Article23. Sanborn BM. Relationship of ion channel activity to control of myometrial calcium. J Soc Gynecol Investig. 2000; 7:4–11.
Article24. Parkington HC, Coleman HA. Excitability in uterine smooth muscle. Front Horm Res. 2001; 27:179–200. PMID: 11450426.
Article25. Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984; 352:685–701. PMID: 6086918.
Article26. Ohmori H. Mechanoelectrical transducer has discrete conductances in the chick vestibular hair cell. Proc Natl Acad Sci U S A. 1984; 81:1888–1891. PMID: 6584923.
Article27. Wellner MC, Isenberg G. Stretch effects on whole-cell currents of guinea-pig urinary bladder myocytes. J Physiol. 1994; 480:439–448. PMID: 7869258.
Article28. Pedersen SF, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol. 2007; 428:183–207. PMID: 17875418.
Article29. Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007; 8:510–521. PMID: 17585304.
Article30. Raoux M, Colomban C, Delmas P, Crest M. The amine-containing cutaneous irritant heptylamine inhibits the volume-regulated anion channel and mobilizes intracellular calcium in normal human epidermal keratinocytes. Mol Pharmacol. 2007; 71:1685–1694. PMID: 17384225.
Article31. Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000; 2:695–702. PMID: 11025659.
Article32. Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000; 103:525–535. PMID: 11081638.
Article33. Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005; 7:179–185. PMID: 15665854.
Article34. Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006; 103:16586–16591. PMID: 17056714.
Article35. Dietrich A, Kalwa H, Storch U, Mederos y Schnitzler M, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007; 455:465–477. PMID: 17647013.
Article36. Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honoré E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 2008; 455:1097–1103. PMID: 17957383.
Article37. Schnitzler JG, Siebert U, Jepson PD, Beineke A, Jauniaux T, Bouquegneau JM, Das K. Harbor porpoise thyroids: histologic investigations and potential interactions with environmental factors. J Wildl Dis. 2008; 44:888–901. PMID: 18957645.
Article38. Dalrymple A, Slater DM, Poston L, Tribe RM. Physiological induction of transient receptor potential canonical proteins, calcium entry channels, in human myometrium: influence of pregnancy, labor, and interleukin-1 beta. J Clin Endocrinol Metab. 2004; 89:1291–1300. PMID: 15001625.39. Jemal I, Soriano S, Conte AL, Morenilla C, Gomis A. G proteincoupled receptor signalling potentiates the osmo-mechanical activation of TRPC5 channels. Pflugers Arch. 2014; 466:1635–1646. PMID: 24177920.
Article40. Gomis A, Soriano S, Belmonte C, Viana F. Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J Physiol. 2008; 586:5633–5649. PMID: 18832422.
Article41. Storch U, Mederos y Schnitzler M, Gudermann T. G protein-mediated stretch reception. Am J Physiol Heart Circ Physiol. 2012; 302:H1241–H1249. PMID: 22227128.
Article42. Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004; 6:499–506. PMID: 15146194.
Article43. Yasuda N, Miura S, Akazawa H, Tanaka T, Qin Y, Kiya Y, Imaizumi S, Fujino M, Ito K, Zou Y, Fukuhara S, Kunimoto S, Fukuzaki K, Sato T, Ge J, Mochizuki N, Nakaya H, Saku K, Komuro I. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep. 2008; 9:179–186. PMID: 18202720.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Ketamine on Rat Myometrial Contractility
- Effect of Opioid Agonists , Diazepam and Ketamine on the Estrous and Pregnant Rat Uteri , in Vitro
- The Role of Transient Receptor Potential Channel in Pain
- Effect of diazepam on the oxytocin induced contraction of the isolated rat uterus
- The effects of etomidate on the contraction of pregnant rat uterine smooth muscle