Korean J Physiol Pharmacol.
2014 Dec;18(6):489-495. 10.4196/kjpp.2014.18.6.489.
Suppression of Peripheral Sympathetic Activity Underlies Protease-Activated Receptor 2-Mediated Hypotension
- Affiliations
-
- 1Department of Physiology, Yonsei University College of Medicine, Seoul 120-752, Korea. sschung@yuhs.ac
- KMID: 2285551
- DOI: http://doi.org/10.4196/kjpp.2014.18.6.489
Abstract
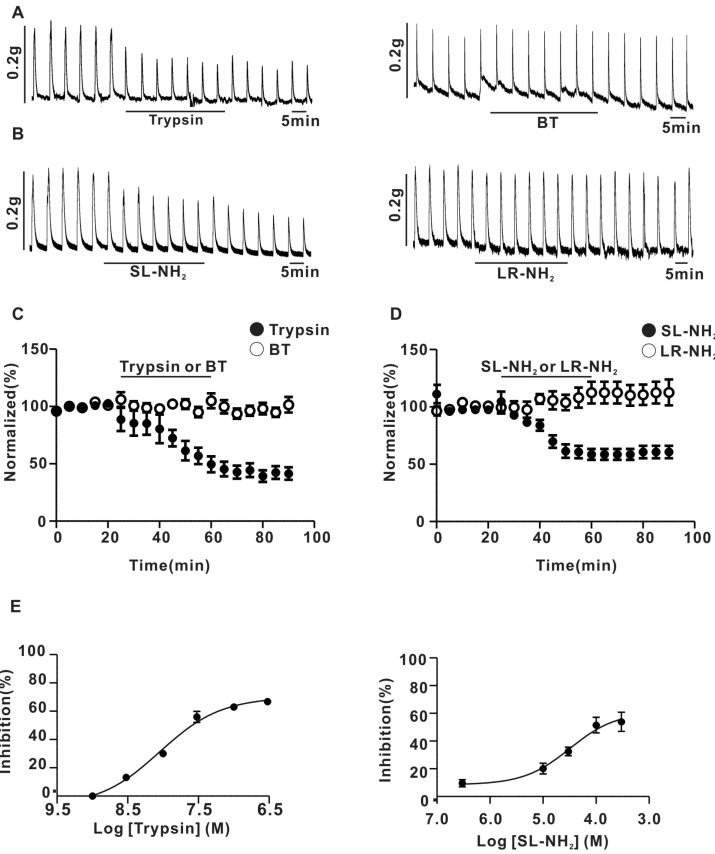
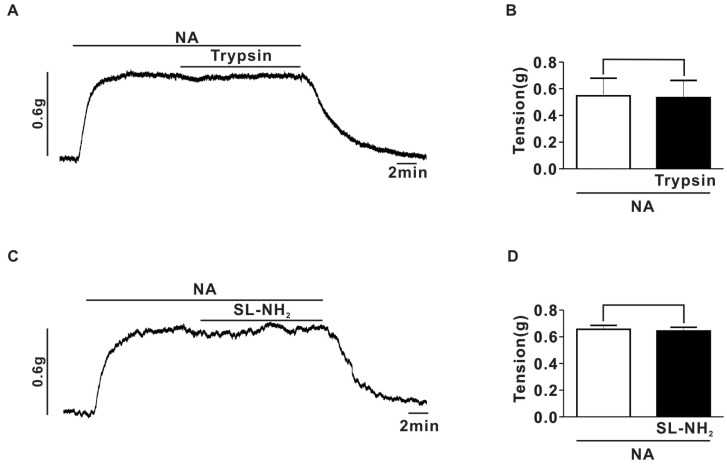
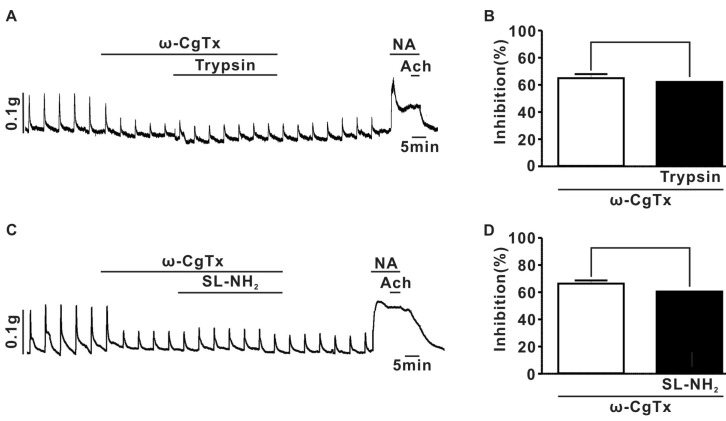
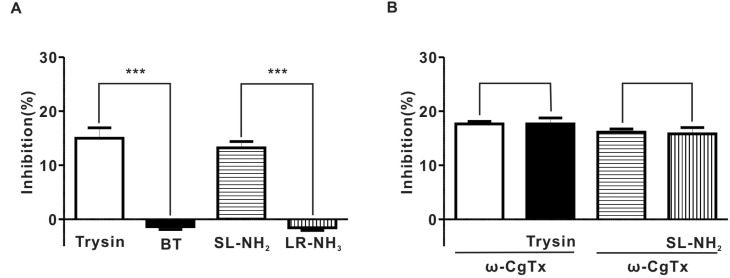
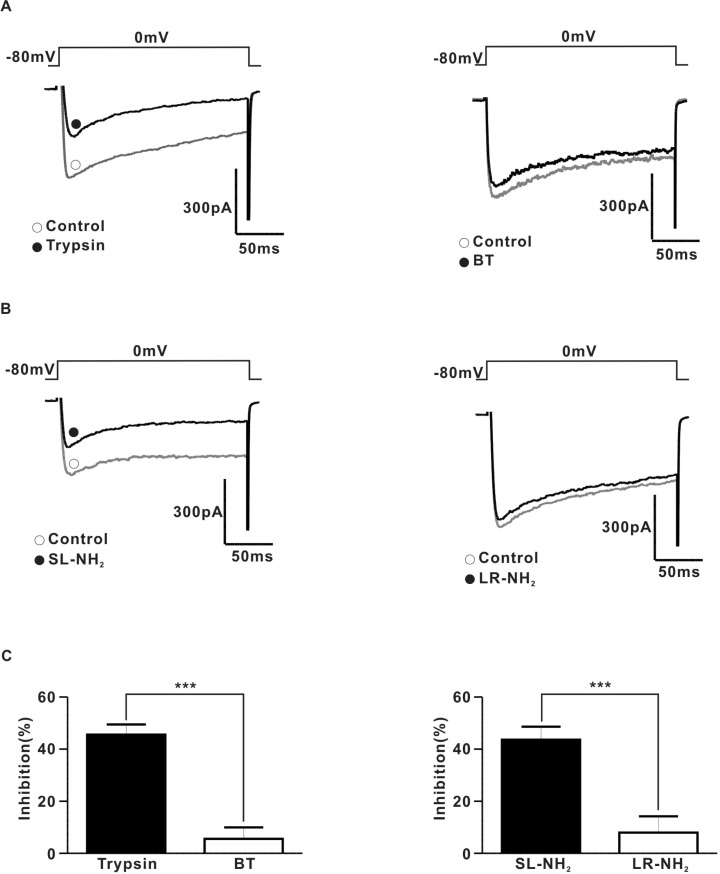
- Protease-activated receptor (PAR)-2 is expressed in endothelial cells and vascular smooth muscle cells. It plays a crucial role in regulating blood pressure via the modulation of peripheral vascular tone. Although some reports have suggested involvement of a neurogenic mechanism in PAR-2-induced hypotension, the accurate mechanism remains to be elucidated. To examine this possibility, we investigated the effect of PAR-2 activation on smooth muscle contraction evoked by electrical field stimulation (EFS) in the superior mesenteric artery. In the present study, PAR-2 agonists suppressed neurogenic contractions evoked by EFS in endothelium-denuded superior mesenteric arterial strips but did not affect contraction elicited by the external application of noradrenaline (NA). However, thrombin, a potent PAR-1 agonist, had no effect on EFS-evoked contraction. Additionally, omega-conotoxin GVIA (CgTx), a selective N-type Ca2+ channel (I(Ca-N)) blocker, significantly inhibited EFS-evoked contraction, and this blockade almost completely occluded the suppression of EFS-evoked contraction by PAR-2 agonists. Finally, PAR-2 agonists suppressed the EFS-evoked overflow of NA in endothelium-denuded rat superior mesenteric arterial strips and this suppression was nearly completely occluded by omega-CgTx. These results suggest that activation of PAR-2 may suppress peripheral sympathetic outflow by modulating activity of I(Ca-N) which are located in peripheral sympathetic nerve terminals, which results in PAR-2-induced hypotension.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Facilitation of serotonin-induced contraction of rat mesenteric artery by ketamine
Sang Woong Park, Hyun Ju Noh, Jung Min Kim, Bokyung Kim, Sung-Il Cho, Yoon Soo Kim, Nam Sik Woo, Sung Hun Kim, Young Min Bae
Korean J Physiol Pharmacol. 2016;20(6):605-611. doi: 10.4196/kjpp.2016.20.6.605.
Reference
-
1. Kawabata A, Kuroda R, Hollenberg MD. Physiology of protease-activated receptors (PARs): involvement of PARs in digestive functions. Nihon Yakurigaku Zasshi. 1999; 114(Suppl 1):173P–179P.
Article2. al-Ani B, Saifeddine M, Hollenberg MD. Detection of functional receptors for the proteinase-activated-receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can J Physiol Pharmacol. 1995; 73:1203–1207. PMID: 8564891.
Article3. Hollenberg MD, Saifeddine M, al-Ani B. Proteinase-activated receptor-2 in rat aorta: structural requirements for agonist activity of receptor-activating peptides. Mol Pharmacol. 1996; 49:229–233. PMID: 8632754.4. Magazine HI, King JM, Srivastava KD. Protease activated receptors modulate aortic vascular tone. Int J Cardiol. 1996; 53(Suppl):S75–S80. PMID: 8793596.
Article5. Saifeddine M, al-Ani B, Cheng CH, Wang L, Hollenberg MD. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br J Pharmacol. 1996; 118:521–530. PMID: 8762073.
Article6. Emilsson K, Wahlestedt C, Sun MK, Nystedt S, Owman C, Sundelin J. Vascular effects of proteinase-activated receptor 2 agonist peptide. J Vasc Res. 1997; 34:267–272. PMID: 9256086.
Article7. Cicala C, Pinto A, Bucci M, Sorrentino R, Walker B, Harriot P, Cruchley A, Kapas S, Howells GL, Cirino G. Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circulation. 1999; 99:2590–2597. PMID: 10330393.8. Cicala C, Morello S, Santagada V, Caliendo G, Sorrentino L, Cirino G. Pharmacological dissection of vascular effects caused by activation of protease-activated receptors 1 and 2 in anesthetized rats. FASEB J. 2001; 15:1433–1435. PMID: 11387248.9. Cheung WM, Andrade-Gordon P, Derian CK, Damiano BP. Receptor-activating peptides distinguish thrombin receptor (PAR-1) and protease activated receptor 2 (PAR-2) mediated hemodynamic responses in vivo. Can J Physiol Pharmacol. 1998; 76:16–25. PMID: 9564545.10. Damiano BP, Cheung WM, Santulli RJ, Fung-Leung WP, Ngo K, Ye RD, Darrow AL, Derian CK, de Garavilla L, Andrade-Gordon P. Cardiovascular responses mediated by proteaseactivated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. J Pharmacol Exp Ther. 1999; 288:671–678. PMID: 9918574.11. Kawabata A, Nakaya Y, Kuroda R, Wakisaka M, Masuko T, Nishikawa H, Kawai K. Involvement of EDHF in the hypotension and increased gastric mucosal blood flow caused by PAR-2 activation in rats. Br J Pharmacol. 2003; 140:247–254. PMID: 12970102.
Article12. Lee CC, Lee YY, Chang CK, Lin MT. Selective inhibition of inducible nitric oxide synthase attenuates renal ischemia and damage in experimental heatstroke. J Pharmacol Sci. 2005; 99:68–76. PMID: 16127242.
Article13. Emilsson V, Arch JR, de Groot RP, Lister CA, Cawthorne MA. Leptin treatment increases suppressors of cytokine signaling in central and peripheral tissues. FEBS Lett. 1999; 455:170–174. PMID: 10428495.
Article14. Molderings GJ, Likungu J, Göthert M. N-Type calcium channels control sympathetic neurotransmission in human heart atrium. Circulation. 2000; 101:403–407. PMID: 10653832.
Article15. Shimosawa T, Takano K, Ando K, Fujita T. Magnesium inhibits norepinephrine release by blocking N-type calcium channels at peripheral sympathetic nerve endings. Hypertension. 2004; 44:897–902. PMID: 15477382.
Article16. Ryu SK, Ahn DS, Cho YE, Choi SK, Kim YH, Morgan KG, Lee YH. Augmented sphingosylphosphorylcholine-induced Ca2+-sensitization of mesenteric artery contraction in spontaneously hypertensive rat. Naunyn Schmiedebergs Arch Pharmacol. 2006; 373:30–36. PMID: 16521007.17. Zygmunt PM, Högestätt ED. Calcium channels at the adrenergic neuroeffector junction in the rabbit ear artery. Naunyn Schmiedebergs Arch Pharmacol. 1993; 347:617–623. PMID: 8395661.
Article18. Ralevic V, Karoon P, Burnstock G. Long-term sensory denervation by neonatal capsaicin treatment augments sympathetic neurotransmission in rat mesenteric arteries by increasing levels of norepinephrine and selectively enhancing postjunctional actions. J Pharmacol Exp Ther. 1995; 274:64–71. PMID: 7616449.19. Kim T, La J, Lee J, Yang I. Effects of nitric oxide on slow waves and spontaneous contraction of guinea pig gastric antral circular muscle. J Pharmacol Sci. 2003; 92:337–347. PMID: 12939518.
Article20. Asano M, Nomura N, Sasaki S, Mishima A. Surgical repair of tetralogy of Fallot with large conus artery. Pediatr Cardiol. 2003; 24:601–603. PMID: 12717594.21. Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994; 91:9208–9212. PMID: 7937743.
Article22. Lamont C, Vainorius E, Wier WG. Purinergic and adrenergic Ca2+ transients during neurogenic contractions of rat mesenteric small arteries. J Physiol. 2003; 549:801–808. PMID: 12740429.23. Sadraei H, Large BJ, Hughes IE. Mechanisms involved in electrically-induced responses of rat seminal vesicles. J Pharm Pharmacol. 1995; 47:665–668. PMID: 8583369.
Article24. Tanaka Y, Mochizuki Y, Tanaka H, Shigenobu K. Significant role of neuronal non-N-type calcium channels in the sympathetic neurogenic contraction of rat mesenteric artery. Br J Pharmacol. 1999; 128:1602–1608. PMID: 10602342.
Article25. Recio P, Orensanz LM, Martnez MP, Navarro-Dorado J, Bustamante S, García-Sacristán A, Prieto D, Hernández M. Noradrenergic vasoconstriction of pig prostatic small arteries. Naunyn Schmiedebergs Arch Pharmacol. 2008; 376:397–406. PMID: 18172615.
Article26. Whorlow SL, Angus JA, Wright CE. Selectivity of omegaconotoxin GVIA for n-type calcium channels in rat isolated small mesenteric arteries. Clin Exp Pharmacol Physiol. 1996; 23:16–21. PMID: 8713491.27. Chien EK, Sweet L, Phillippe M, Marietti S, Kim TT, Wolff DA, Thomas L, Bieber E. Protease-activated receptor isoform expression in pregnant and nonpregnant rat myometrial tissue. J Soc Gynecol Investig. 2003; 10:460–468.
Article28. Hwa JJ, Ghibaudi L, Williams P, Chintala M, Zhang R, Chatterjee M, Sybertz E. Evidence for the presence of a proteinase-activated receptor distinct from the thrombin receptor in vascular endothelial cells. Circ Res. 1996; 78:581–588. PMID: 8635215.
Article29. Hamilton JR, Nguyen PB, Cocks TM. Atypical protease-activated receptor mediates endothelium-dependent relaxation of human coronary arteries. Circ Res. 1998; 82:1306–1311. PMID: 9648727.
Article30. Roy SS, Saifeddine M, Loutzenhiser R, Triggle CR, Hollenberg MD. Dual endothelium-dependent vascular activities of proteinase-activated receptor-2-activating peptides: evidence for receptor heterogeneity. Br J Pharmacol. 1998; 123:1434–1440. PMID: 9579740.
Article31. Sobey CG, Moffatt JD, Cocks TM. Evidence for selective effects of chronic hypertension on cerebral artery vasodilatation to protease-activated receptor-2 activation. Stroke. 1999; 30:1933–1940. discussion 1941. PMID: 10471447.
Article32. McGuire JJ. Proteinase-activated Receptor 2 (PAR2): a challenging new target for treatment of vascular diseases. Curr Pharm Des. 2004; 10:2769–2778. PMID: 15320742.
Article33. Camerer E, Cornelissen I, Kataoka H, Duong DN, Zheng YW, Coughlin SR. Roles of protease-activated receptors in a mouse model of endotoxemia. Blood. 2006; 107:3912–3921. PMID: 16434493.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thrombin Induced Apoptosis through Calcium-Mediated Activation of Cytosolic Phospholipase A2 in Intestinal Myofibroblasts
- Role of Coagulation Factor 2 Receptor during Respiratory Pneumococcal Infections
- Changes of Electrophysiologic Properties of Dorsal Root Ganglion Cells in Peripheral Nerve-injured Rats
- Protease and Protease-Activated Receptor-2 Signaling in the Pathogenesis of Atopic Dermatitis
- Epidermal Proteinase-Activated Receptor-2 Expression is Increased in End-Stage Renal Disease Patients with Pruritus: A Pilot Study