Korean J Physiol Pharmacol.
2014 Apr;18(2):121-127. 10.4196/kjpp.2014.18.2.121.
Effects of Acupuncture Stimulation at Different Acupoints on Formalin-Induced Pain in Rats
- Affiliations
-
- 1Department of Physiology, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul 120-752, Korea. bhlee@yuhs.ac
- 2Department of Anesthesiology and Pain Medicine and Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 3Acupuncture and Meridian Science Research Center, Kyung Hee University, Seoul 130-701, Korea.
- KMID: 2285500
- DOI: http://doi.org/10.4196/kjpp.2014.18.2.121
Abstract
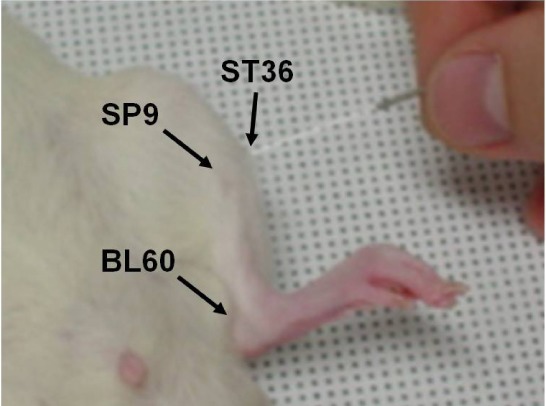
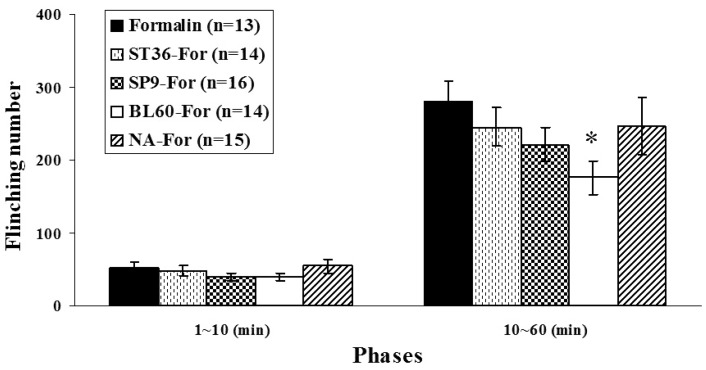
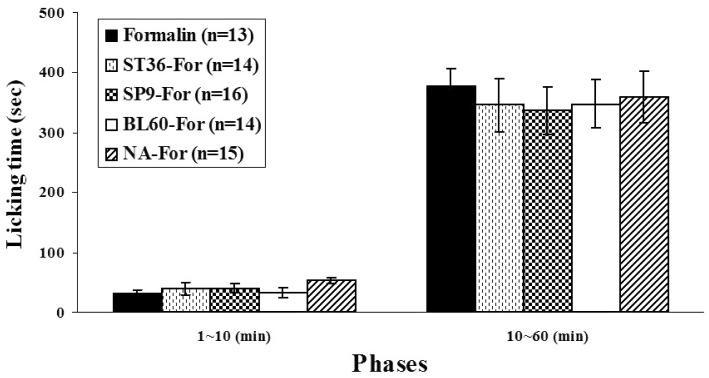
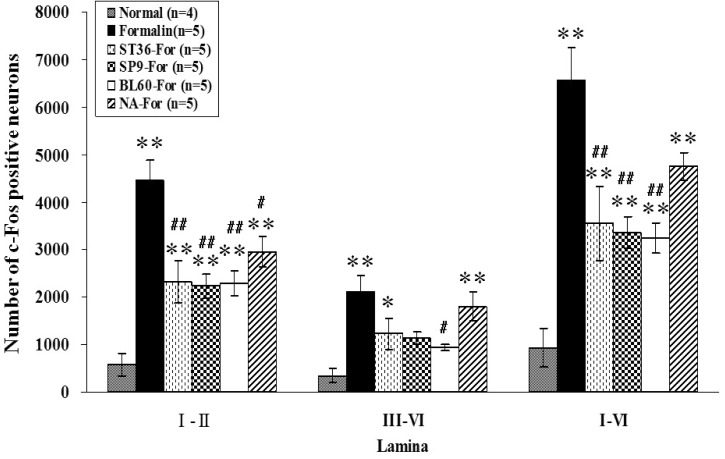
- Acupuncture is the process of stimulating skin regions called meridians or acupoints and has been used to treat pain-related symptoms. However, the pain-relieving effects of acupuncture may be different depending on acupoints. In the present study, the effects of acupuncture on behavioral responses and c-Fos expression were evaluated using a formalin test in male Sprague-Dawley rats in order to clarify the analgesic effects of three different acupoints. Each rat received manual acupuncture at the ST36 (Zusanli), SP9 (Yinlingquan) or BL60 (Kunlun) acupoint before formalin injection. Flinching and licking behaviors were counted by two blinded investigators. Fos-like immunoreactivity was examined by immunohistochemistry in the rat spinal cord. Manual acupuncture treatment at BL60 acupoint showed significant inhibition in flinching behavior but not in licking. Manual acupuncture at ST36 or SP9 tended to inhibit flinching and licking behaviors but the effects were not statistically significant. The acupuncture at ST36, SP9, or BL60 reduced c-Fos expression as compared with the control group. These results suggest that acupuncture especially at the BL60 acupoint is more effective in relieving inflammatory pain than other acupoints.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Antinociceptive Effects of Transcytosed Botulinum Neurotoxin Type A on Trigeminal Nociception in Rats
Hye-Jin Kim, Geun-Woo Lee, Min-Ji Kim, Kui-Ye Yang, Seong-Taek Kim, Yong-Cheol Bae, Dong-Kuk Ahn
Korean J Physiol Pharmacol. 2015;19(4):349-355. doi: 10.4196/kjpp.2015.19.4.349.
Reference
-
1. Vincent CA, Richardson PH. The evaluation of therapeutic acupuncture: concepts and methods. Pain. 1986; 24:1–13. PMID: 3513094.
Article2. Ahmed S, Anuntiyo J, Malemud CJ, Haqqi TM. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evid Based Complement Alternat Med. 2005; 2:301–308. PMID: 16136208.
Article3. Berman BM, Swyers JP, Ezzo J. The evidence for acupuncture as a treatment for rheumatologic conditions. Rheum Dis Clin North Am. 2000; 26:103–115. PMID: 10680198.
Article4. Diehl DL, Kaplan G, Coulter I, Glik D, Hurwitz EL. Use of acupuncture by American physicians. J Altern Complement Med. 1997; 3:119–126. PMID: 9395701.
Article5. Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998; 280:1569–1575. PMID: 9820257.6. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993; 328:246–252. PMID: 8418405.7. Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. 2002; 136:374–383. PMID: 11874310.
Article8. Cha MH, Bai SJ, Lee KH, Cho ZH, Kim YB, Lee HJ, Lee BH. Acute electroacupuncture inhibits nitric oxide synthase expression in the spinal cord of neuropathic rats. Neurol Res. 2010; 32(Suppl 1):96–100. PMID: 20034455.
Article9. Cha MH, Choi JS, Bai SJ, Shim I, Lee HJ, Choi SM, Lee BH. Antiallodynic effects of acupuncture in neuropathic rats. Yonsei Med J. 2006; 47:359–366. PMID: 16807985.
Article10. Chang KH, Won R, Shim I, Lee H, Lee BH. Effects of Electroacupuncture at BL60 on Formalin-Induced Pain in Rats. Evid Based Complement Alternat Med. 2012; 2012:324039. PMID: 22550540.
Article11. Hao S, Takahata O, Iwasaki H. Electroacupuncture potentiates the antinociceptive effect of intrathecal endomorphin-1 in the rat formalin test. Neurosci Lett. 2000; 287:9–12. PMID: 10841978.
Article12. Kim SJ, Chung ES, Lee JH, Lee CH, Kim SK, Lee HJ, Bae H. Electroacupuncture analgesia is improved by adenoviral gene transfer of dopamine beta-hydroxylase into the hypothalamus of rats. Korean J Physiol Pharmacol. 2013; 17:505–510. PMID: 24381499.
Article13. Oh JH, Bai SJ, Cho ZH, Han HC, Min SS, Shim I, Lee HJ, Lee H, Lee BH. Pain-relieving effects of acupuncture and electroacupuncture in an animal model of arthritic pain. Int J Neurosci. 2006; 116:1139–1156. PMID: 16923683.
Article14. Shou Y, Yang Y, Xu MS, Zhao YQ, Ge LB, Zhang BM. Electroacupuncture inhibition of hyperalgesia in rats with adjuvant arthritis: involvement of cannabinoid receptor 1 and dopamine receptor subtypes in striatum. Evid Based Complement Alternat Med. 2013; 2013:393460. PMID: 23762129.
Article15. Wen YR, Yeh GC, Shyu BC, Ling QD, Wang KC, Chen TL, Sun WZ. A minimal stress model for the assessment of electroacupuncture analgesia in rats under halothane. Eur J Pain. 2007; 11:733–742. PMID: 17218131.
Article16. Wheeler-Aceto H, Porreca F, Cowan A. The rat paw formalin test: comparison of noxious agents. Pain. 1990; 40:229–238. PMID: 2308768.
Article17. Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992; 51:5–17. PMID: 1454405.
Article18. Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977; 4:161–174. PMID: 564014.
Article19. Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989; 38:347–352. PMID: 2478947.
Article20. Kwak Y, Rhyu MR, Bai SJ, Sa YH, Kwon MJ, Lee BH. c-Fos expression in the nucleus of the solitary tract in response to salt stimulation in rats. Korean J Physiol Pharmacol. 2011; 15:437–443. PMID: 22359483.
Article21. Schaap MW, van Oostrom H, Doornenbal A, van't Klooster J, Baars AM, Arndt SS, Hellebrekers LJ. Nociception and conditioned fear in rats: strains matter. PLoS One. 2013; 8:e83339. PMID: 24376690.
Article22. Tobaldini G, de Siqueira BA, Lima MM, Tambeli CH, Fischer L. Ascending nociceptive control contributes to the anti-nociceptive effect of acupuncture in a rat model of acute pain. J Pain. 2014; pii: S1526-5900(14)00021-2.
Article23. Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990; 296:517–530. PMID: 2113539.24. Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987; 328:632–634. PMID: 3112583.
Article25. Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998; 45:1–8. PMID: 9434195.
Article26. Li WM, Cui KM, Li N, Gu QB, Schwarz W, Ding GH, Wu GC. Analgesic effect of electroacupuncture on complete Freund's adjuvant-induced inflammatory pain in mice: a model of antipain treatment by acupuncture in mice. Jpn J Physiol. 2005; 55:339–344. PMID: 16356296.
Article27. Kagitani F, Uchida S, Hotta H, Aikawa Y. Manual acupuncture needle stimulation of the rat hindlimb activates groups I, II, III and IV single afferent nerve fibers in the dorsal spinal roots. Jpn J Physiol. 2005; 55:149–155. PMID: 15992454.
Article28. Kim JH, Gwak YS, Lee I, Sohn IC, Kim MS, Choi DO, Baek DB, Park BR. Antinociceptive effects of heterotopic electroacupuncture in formalin-induced pain. Am J Chin Med. 2006; 34:565–574. PMID: 16883628.
Article29. Chang FC, Tsai HY, Yu MC, Yi PL, Lin JG. The central serotonergic system mediates the analgesic effect of electroacupuncture on ZUSANLI (ST36) acupoints. J Biomed Sci. 2004; 11:179–185. PMID: 14966368.30. Chang CJ, Huang ST, Hsu K, Lin A, Stoller ML, Lue TF. Electroacupuncture decreases c-fos expression in the spinal cord induced by noxious stimulation of the rat bladder. J Urol. 1998; 160:2274–2279. PMID: 9817383.
Article31. Yu XJ, Zhan R, Huang H, Ding GH. Analysis on the difference of afferent mechanism of analgesic signals from manual acupuncture and electroacupuncture of "Zusanli" (ST 36). Zhen Ci Yan Jiu. 2008; 33:310–315. PMID: 19097502.32. Yuan B, Pang T, Liu X. Response properties of SI cortical neurons to electro-acupuncture and manual acupuncture in the rat. Zhen Ci Yan Jiu. 1991; 16:79–86. PMID: 1914135.33. Leung AY, Park J, Schulteis G, Duann JR, Yaksh T. The electrophysiology of de qi sensations. J Altern Complement Med. 2006; 12:743–750. PMID: 17034280.
Article34. Kim JH, Min BI, Schmidt D, Lee HJ, Park DS. The difference between electroacupuncture only and electroacupuncture with manipulation on analgesia in rats. Neurosci Lett. 2000; 279:149–152. PMID: 10688051.
Article35. Kim SK, Park JH, Bae SJ, Kim JH, Hwang BG, Min BI, Park DS, Na HS. Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: mediation by spinal adrenergic and serotonergic receptors. Exp Neurol. 2005; 195:430–436. PMID: 16054138.
Article36. Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biol Psychiatry. 1998; 44:129–138. PMID: 9646895.
Article37. Coderre TJ, Vaccarino AL, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res. 1990; 535:155–158. PMID: 2292020.
Article38. Presley RW, Menétrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990; 10:323–335. PMID: 1688935.
Article39. Hathaway CB, Hu JW, Bereiter DA. Distribution of Fos-like immunoreactivity in the caudal brainstem of the rat following noxious chemical stimulation of the temporomandibular joint. J Comp Neurol. 1995; 356:444–456. PMID: 7642805.
Article40. Lee IO, Jeong YS. Effects of different concentrations of formalin on paw edema and pain behaviors in rats. J Korean Med Sci. 2002; 17:81–85. PMID: 11850594.
Article41. Gogas KR, Presley RW, Levine JD, Basbaum AI. The antinociceptive action of supraspinal opioids results from an increase in descending inhibitory control: correlation of nociceptive behavior and c-fos expression. Neuroscience. 1991; 42:617–628. PMID: 1659673.
Article42. Jones SL. Noradrenergic modulation of noxious heat-evoked fos-like immunoreactivity in the dorsal horn of the rat sacral spinal cord. J Comp Neurol. 1992; 325:435–445. PMID: 1360018.
Article43. Chapman V, Honoré P, Buritova J, Besson JM. The contribution of NMDA receptor activation to spinal c-Fos expression in a model of inflammatory pain. Br J Pharmacol. 1995; 116:1628–1634. PMID: 8564229.
Article44. Peets JM, Pomeranz B. CXBK mice deficient in opiate receptors show poor electroacupuncture analgesia. Nature. 1978; 273:675–676. PMID: 208000.
Article45. Peng CH, Yang MM, Kok SH, Woo YK. Endorphin release: a possible mechanism of acupuncture analgesia. Comp Med East West. 1978; 6:57–60. PMID: 710079.
Article46. Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978; 4:451–462. PMID: 216303.
Article47. Ernst M, Lee MH. Sympathetic vasomotor changes induced by manual and electrical acupuncture of the Hoku point visualized by thermography. Pain. 1985; 21:25–33. PMID: 3982836.
Article48. Cheng RS, Pomeranz B. Monoaminergic mechanism of electroacupuncture analgesia. Brain Res. 1981; 215:77–92. PMID: 6114781.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Simultaneous Electroacupuncture on Ankle Sprain Pain in Rats
- Effective Acupoint of Electroacupuncture on Ankle-sprained Pain in Rats
- Antiallodynic Effects of Acupuncture in Neuropathic Rats
- Effect of Acupuncture on Regional Cerebral Blood Flow at Acupoints GV 20 , GV. 26 , LI. 4 , ST. 36 , SP. 6 Evaluated by Tc-99m ECD Brain SPECT
- Transcutaneous electrical nerve stimulation, acupuncture, and spinal cord stimulation on neuropathic, inflammatory and, noninflammatory pain in rat models