Korean J Physiol Pharmacol.
2014 Apr;18(2):103-108. 10.4196/kjpp.2014.18.2.103.
Push-in Head Restraining Apparatus for Intracranial Self Stimulation Tasks in Rats
- Affiliations
-
- 1Department of Pharmacology, School of Medicine and Brain Science and Engineering Institute, Kyungpook National University, Daegu 702-911, Korea. mglee@knu.ac.kr
- 2Department of Pharmacology, School of Dentistry and Brain Science and Engineering Institute, Kyungpook National University, Daegu 700-412, Korea.
- KMID: 2285498
- DOI: http://doi.org/10.4196/kjpp.2014.18.2.103
Abstract
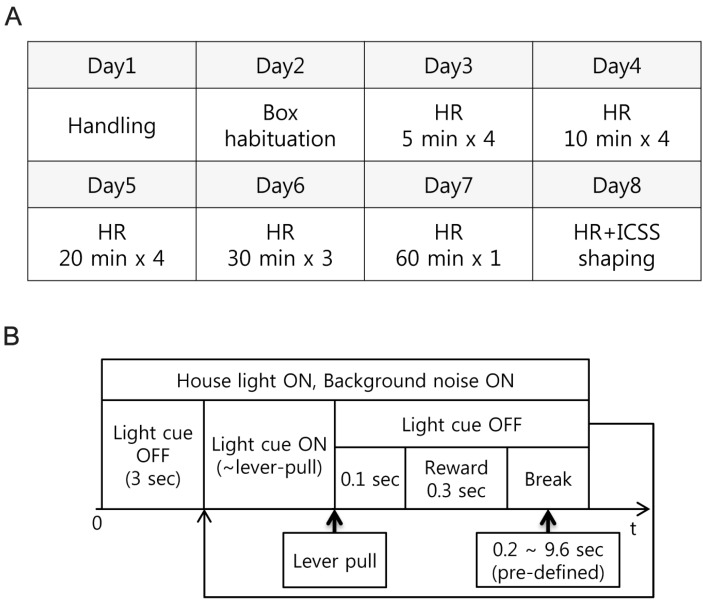
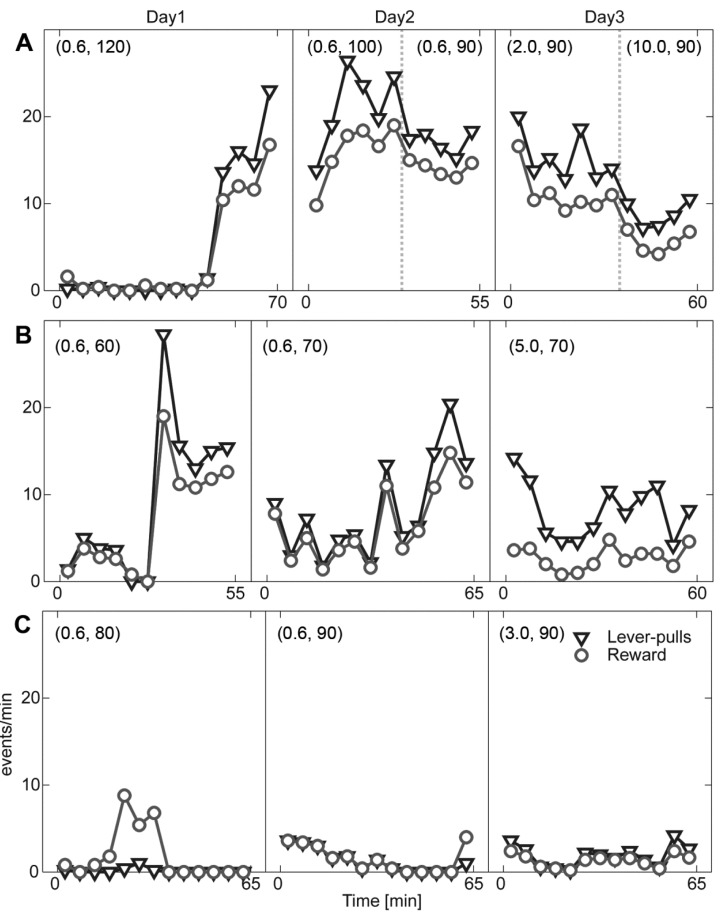
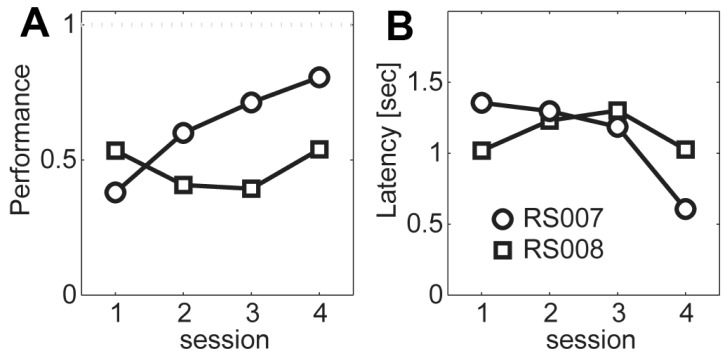
- Head restraining is an experimental technique that firmly secures the animal's head to a fixation apparatus for the precise control and sensing of behaviors. However, procedural and surgical difficulties and limitations have been obstructing the use of the technique in neurophysiological and behavioral experiments. Here, we propose a novel design of the head-restraining apparatus which is easy to develop and convenient for practical use. Head restraining procedure can be completed by sliding the head mounter, which is molded by dental cement during implantation surgery, into the port, which serves as matching guide rails for the mounter, of the fixation bar. So neither skull-attached plates nor screws for fixation are needed. We performed intracranial self stimulation experiment in rats using the newly designed device. Rats were habituated to acclimatize the head-restraint environment and trained to discriminate two spatially distinguished cues using a customized push-pull lever as an operandum. Direct electrical stimulation into the medial forebrain bundle served as reward. We confirmed that head restraining was stable throughout experiments and rats were able to learn to manipulate the lever after successful habituation. Our experimental framework might help precise control or sensing of behavior under head fixed rats using direct electrical brain stimulation as a reward.
MeSH Terms
Figure
Reference
-
1. Isomura Y, Harukuni R, Takekawa T, Aizawa H, Fukai T. Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat Neurosci. 2009; 12:1586–1593. PMID: 19898469.
Article2. Topchiy IA, Wood RM, Peterson B, Navas JA, Rojas MJ, Rector DM. Conditioned lick behavior and evoked responses using whisker twitches in head restrained rats. Behav Brain Res. 2009; 197:16–23. PMID: 18718491.
Article3. O'Connor DH, Clack NG, Huber D, Komiyama T, Myers EW, Svoboda K. Vibrissa-based object localization in head-fixed mice. J Neurosci. 2010; 30:1947–1967. PMID: 20130203.4. Mayrhofer JM, Skreb V, von der, Musall S, Weber B, Haiss F. Novel two-alternative forced choice paradigm for bilateral vibrotactile whisker frequency discrimination in head-fixed mice and rats. J Neurophysiol. 2013; 109:273–284. PMID: 23054598.
Article5. Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007; 56:43–57. PMID: 17920014.
Article6. Schwarz C, Hentschke H, Butovas S, Haiss F, Stüttgen MC, Gerdjikov TV, Bergner CG, Waiblinger C. The head-fixed behaving rat--procedures and pitfalls. Somatosens Mot Res. 2010; 27:131–148. PMID: 20954892.
Article7. Lee MG, Manns ID, Alonso A, Jones BE. Sleep-wake related discharge properties of basal forebrain neurons recorded with micropipettes in head-fixed rats. J Neurophysiol. 2004; 92:1182–1198. PMID: 15028746.
Article8. Parry TJ, McElligott JG. A method for restraining awake rats using head immobilization. Physiol Behav. 1993; 53:1011–1015. PMID: 8099749.
Article9. Bryant JL, Roy S, Heck DH. A technique for stereotaxic recordings of neuronal activity in awake, head-restrained mice. J Neurosci Methods. 2009; 178:75–79. PMID: 19073214.
Article10. Haiss F, Butovas S, Schwarz C. A miniaturized chronic microelectrode drive for awake behaving head restrained mice and rats. J Neurosci Methods. 2010; 187:67–72. PMID: 20036690.
Article11. Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996; 19:319–340. PMID: 8833446.
Article12. Carlezon WA Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007; 2:2987–2995. PMID: 18007634.
Article13. Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. 4th ed. New York: Academic Press;1998.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Electrical Stimulation for the Treatment of Tinnitus
- Treatment of upper ureteral stone with ESWL: push back effect
- Intracranial Pressure and Experimental Model of Diffuse Brain Injury in Rats
- Changes of the Vital Sign, Cerebral Perfusion Pressure and Intracranial Pressure in Variable Degree of Head Elevation
- Current Tasks and Appropriate Tasks of Clinical Research Coordinators in Korea