Korean J Physiol Pharmacol.
2009 Dec;13(6):517-526. 10.4196/kjpp.2009.13.6.517.
Polyphenols of Rubus coreanum Inhibit Catecholamine Secretion from the Perfused Adrenal Medulla of SHRs
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chosun University, Gwangju 501-759, Korea.
- 2Department of Pharmacology, Chosun University, Gwangju 501-759, Korea. dylim@chosun.ac.kr
- 3Department of Bio-materials, DNA Repair Research Center, Chosun University, Gwangju 501-759, Korea.
- KMID: 2285390
- DOI: http://doi.org/10.4196/kjpp.2009.13.6.517
Abstract
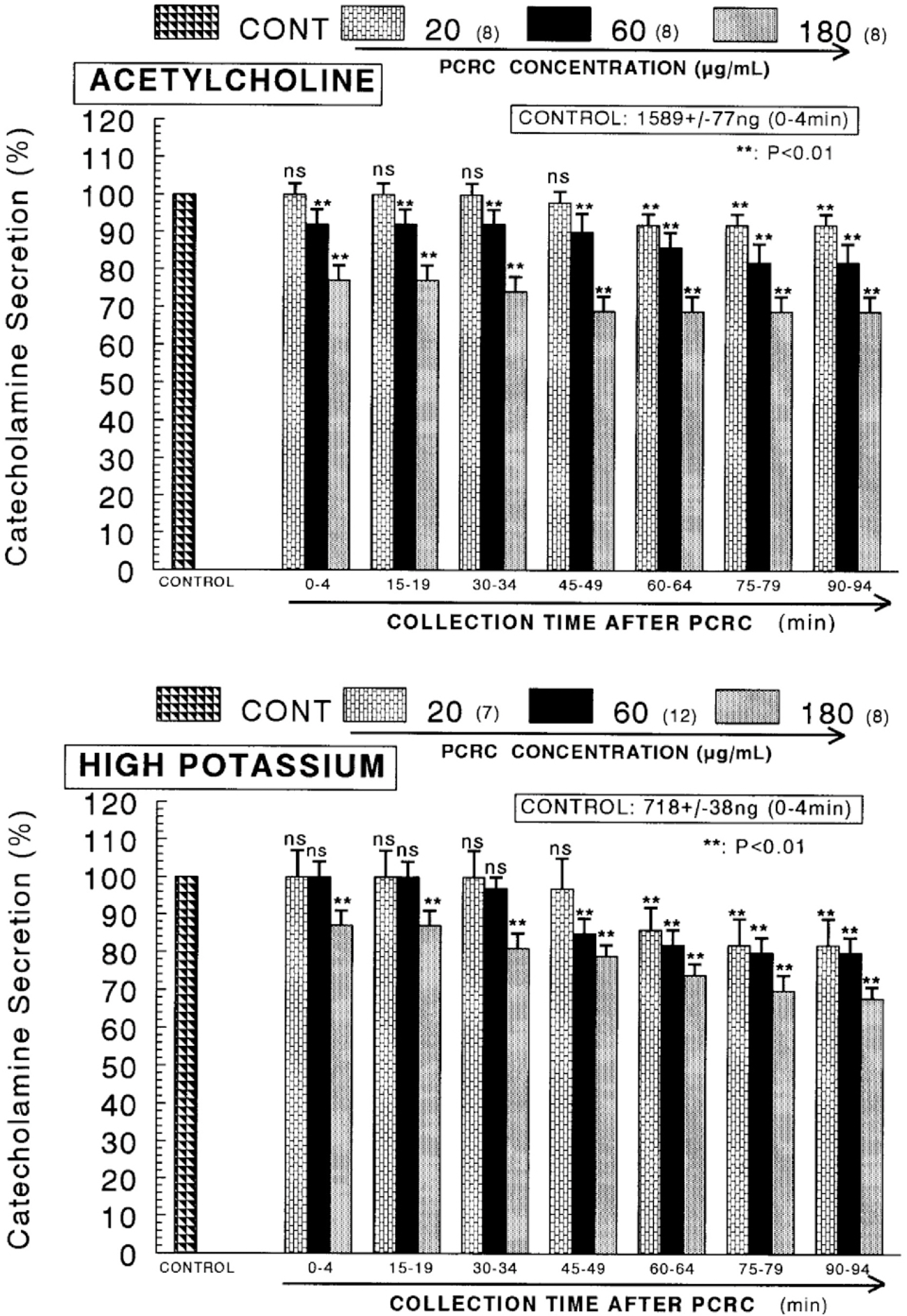
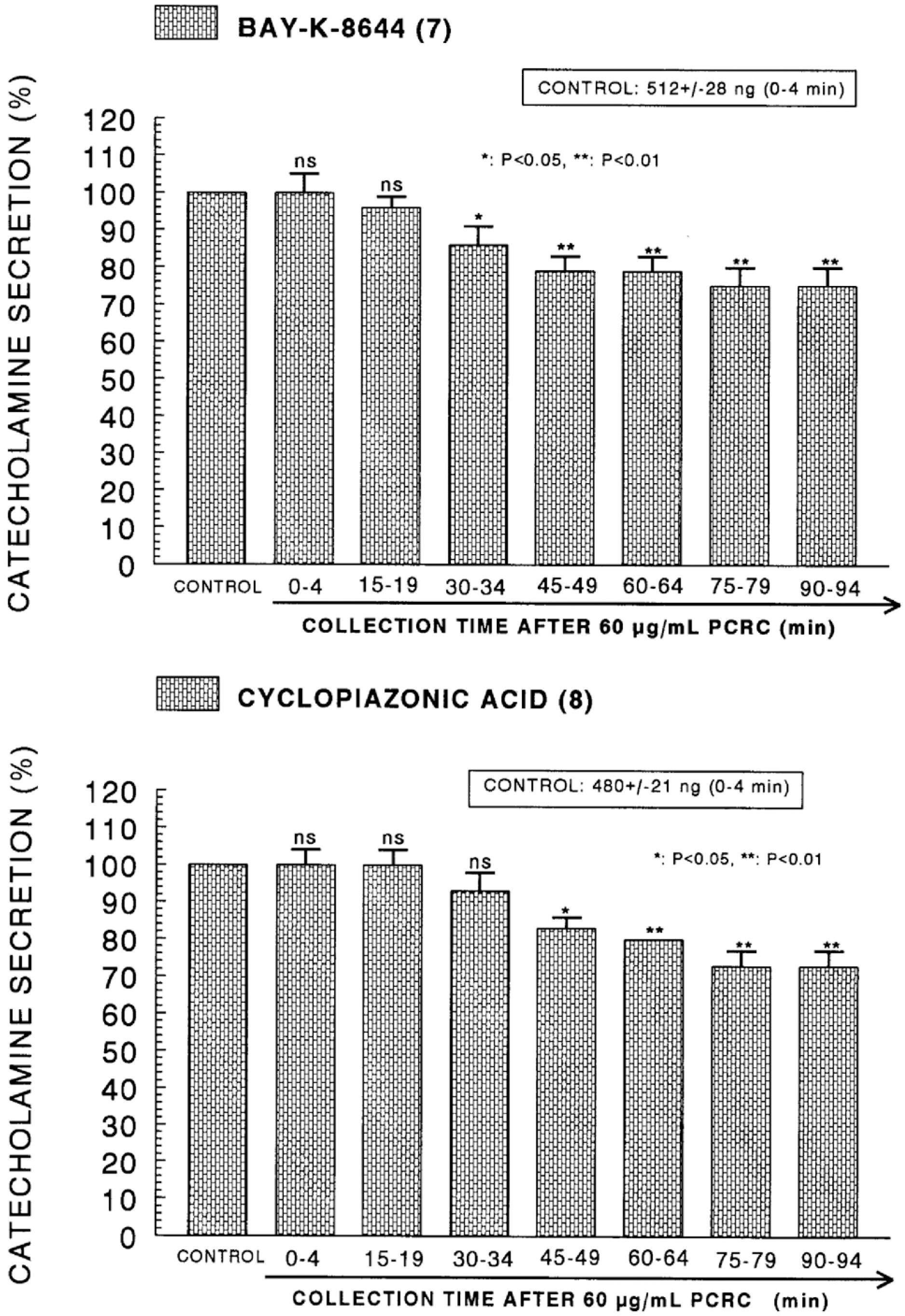
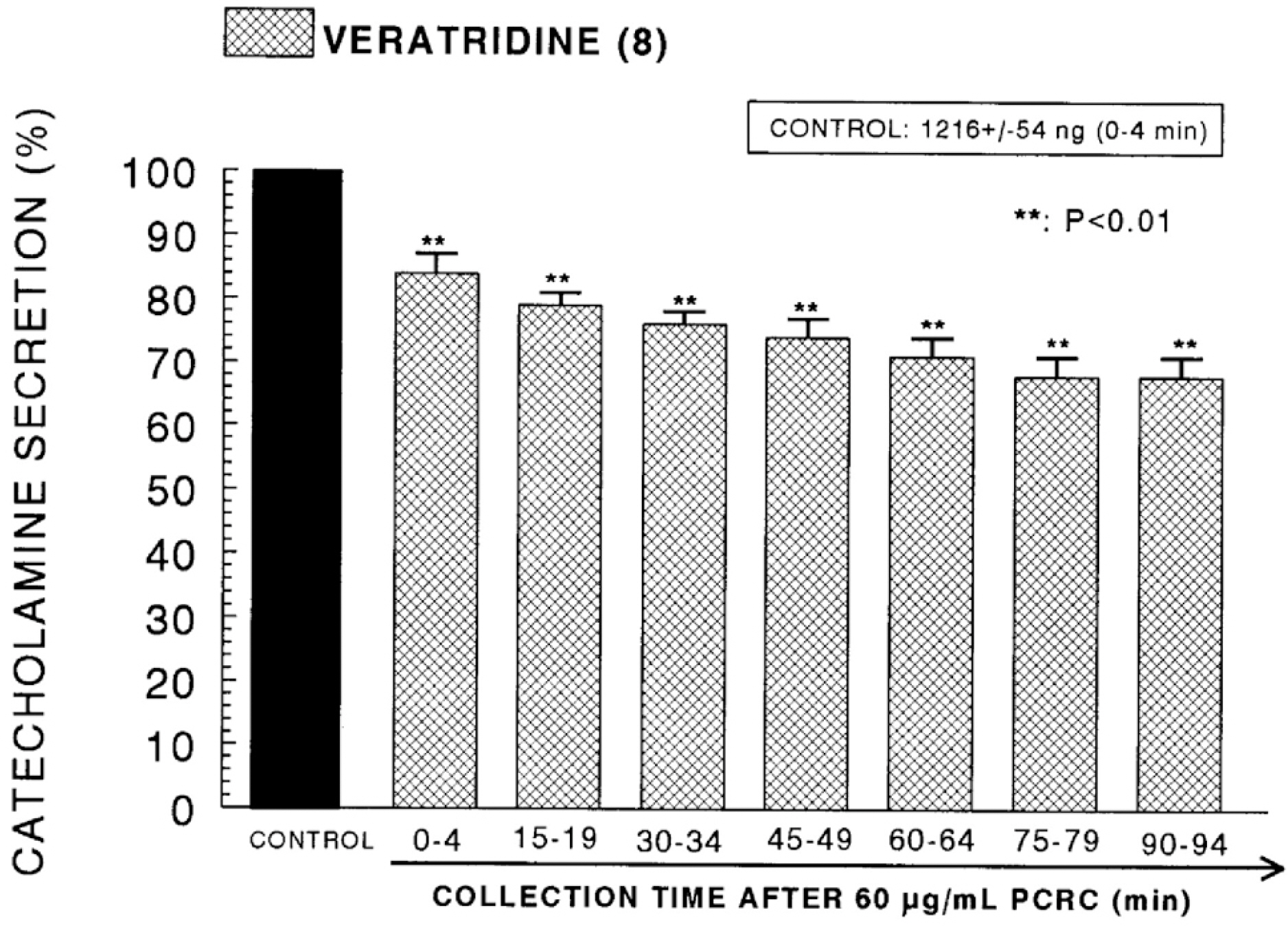
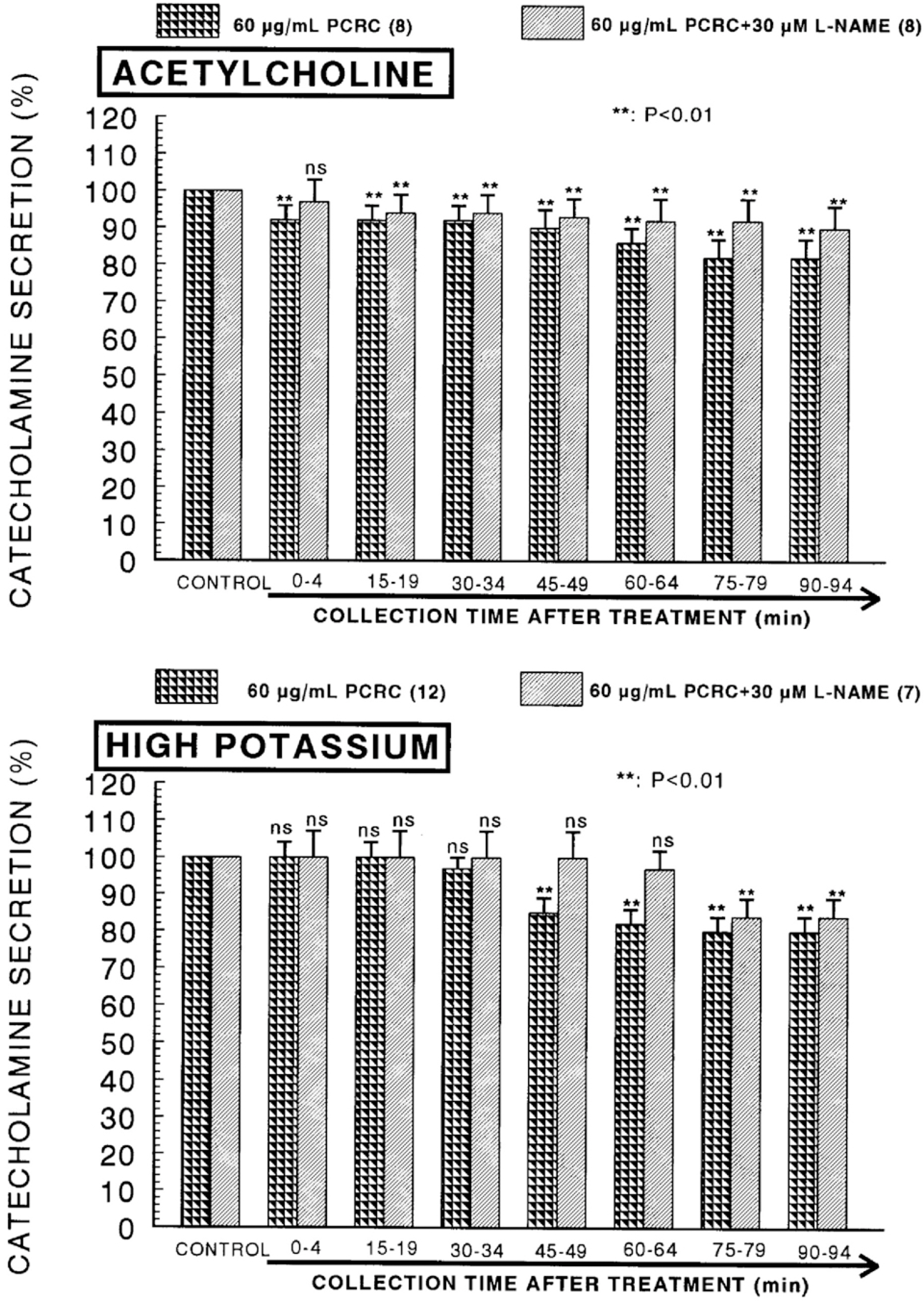
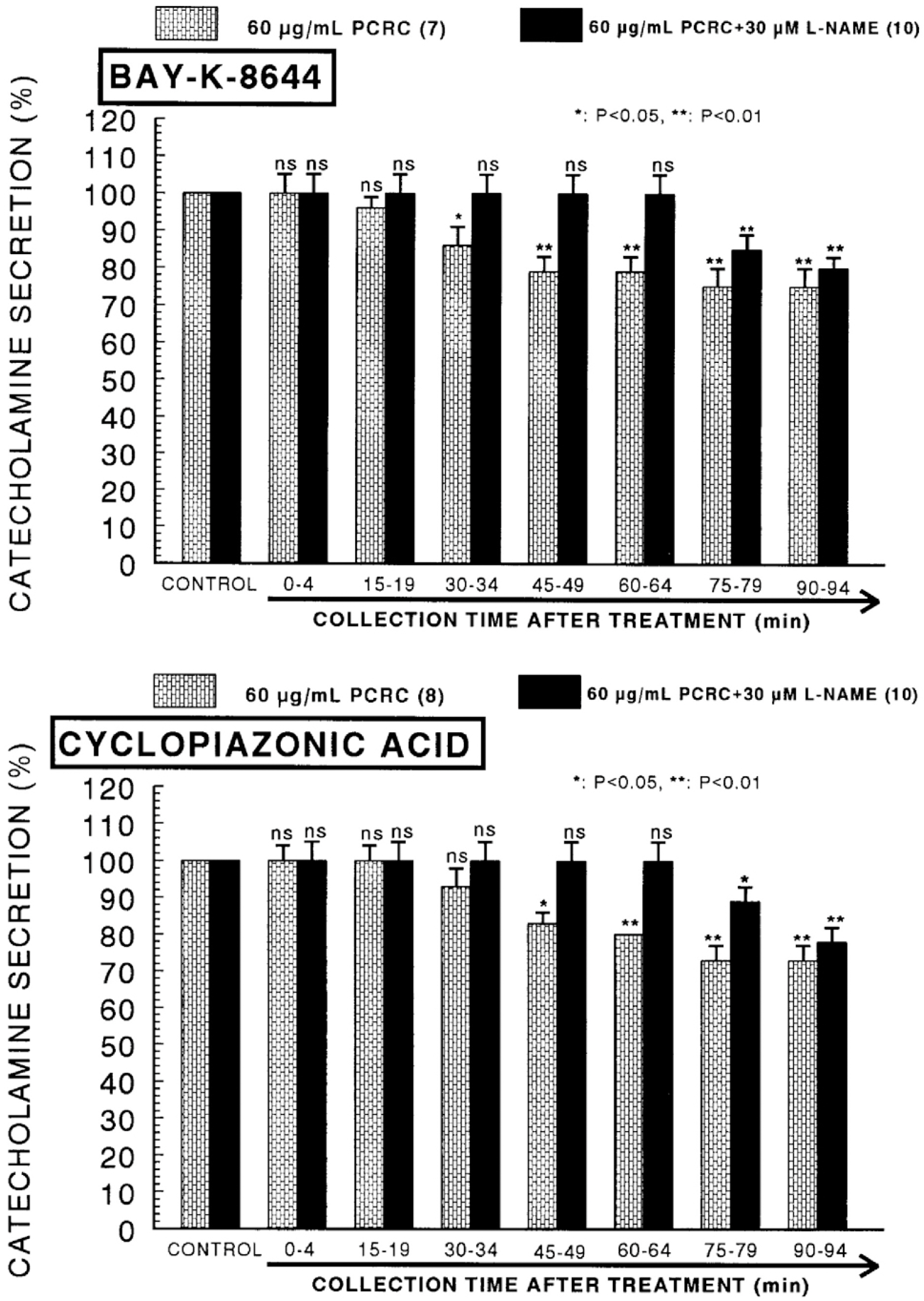
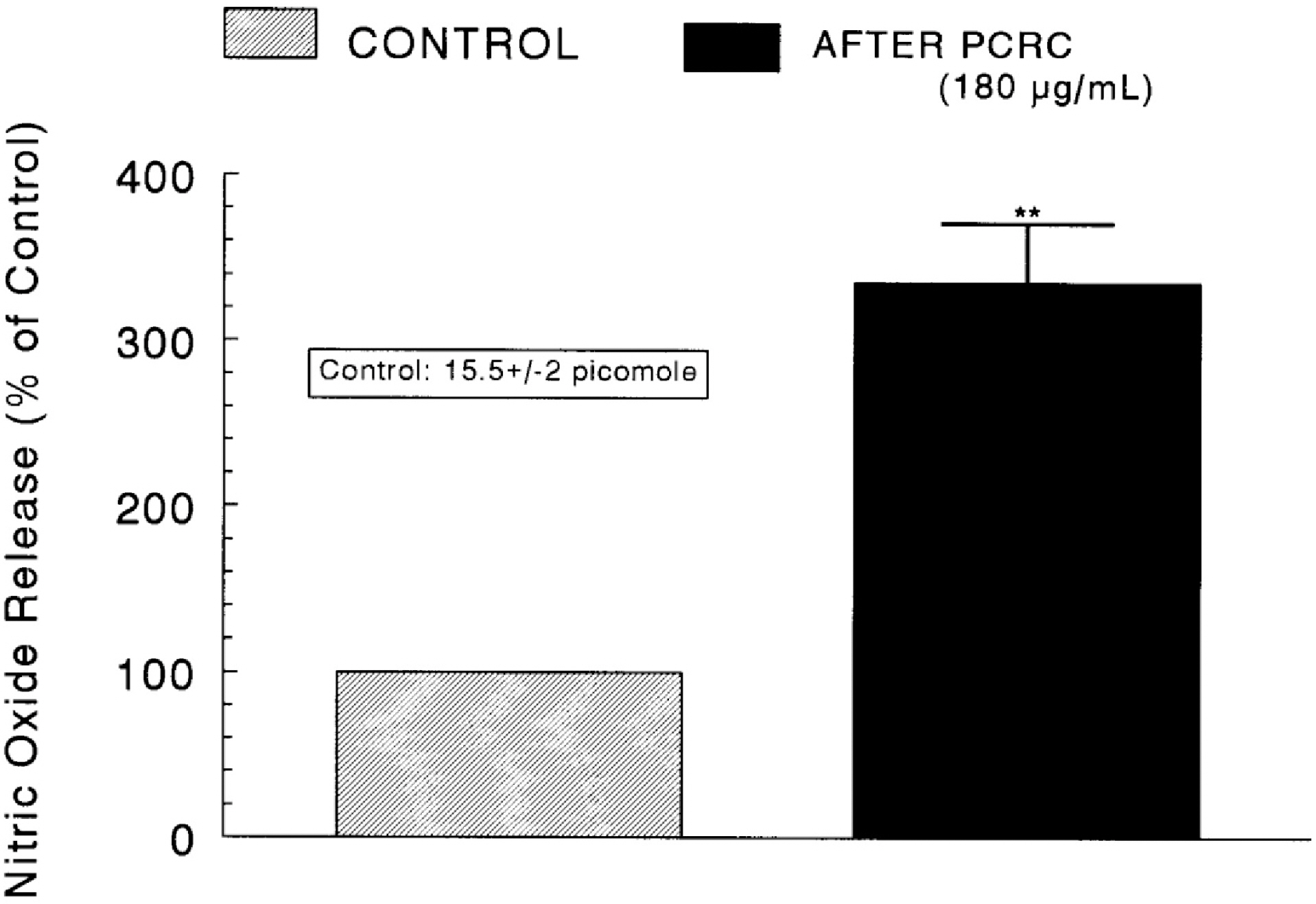
- The present study was attempted to investigate whether polyphenolic compounds isolated from wine, which is brewed from Rubus coreanum Miquel (PCRC), may affect the release of catecholamines (CA) from the isolated perfused adrenal medulla of the spontaneously hypertensive rats (SHRs), and to establish its mechanism of action. PCRC (20~180 microgram/ml) perfused into an adrenal vein for 90 min relatively dose-dependently inhibited the CA secretory responses to ACh (5.32 mM), high K+ (56 mM), DMPP (100 micrometer) and McN-A-343 (100 micrometer). PCRC itself did not affect basal CA secretion (data not shown). Also, in the presence of PCRC (60 microgram/ml), the CA secretory responses to veratridine (a selective Na+ channel activator (10 micrometer), Bay-K-8644 (a L-type dihydropyridine Ca2+ channel activator, 10 micrometer), and cyclopiazonic acid (a cytoplasmic Ca2+ -ATPase inhibitor, 10 micrometer) were significantly reduced, respectively. In the simultaneous presence of PCRC (60 microgram/ml) and L-NAME (an inhibitor of NO synthase, 30 micrometer), the inhibitory responses of PCRC on the CA secretion evoked by ACh, high K+, DMPP, and Bay-K-8644 were considerably recovered to the extent of the corresponding control secretion compared with that of PCRC-treatment alone. The level of NO released from adrenal medulla after the treatment of PCRC (60 microgram/ml) was greatly elevated compared with the corresponding basal level. Taken together, these results demonstrate that PCRC inhibits the CA secretion from the isolated perfused adrenal medulla of the SHRs evoked by stimulation of cholinergic receptors as well as by direct membrane-depolarization. It seems that this inhibitory effect of PCRC is mediated by blocking the influx of calcium and sodium into the adrenal medullary chromaffin cells of the SHRs as well as by inhibition of Ca2+ release from the cytoplasmic calcium store at least partly through the increased NO production due to the activation of NO synthase.
MeSH Terms
-
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Adrenal Medulla
Calcium
Catecholamines
Chromaffin Cells
Cytoplasm
Dihydropyridines
Dimethylphenylpiperazinium Iodide
Indoles
NG-Nitroarginine Methyl Ester
Nitric Oxide
Nitric Oxide Synthase
Polyphenols
Rats, Inbred SHR
Receptors, Cholinergic
Sodium
Veins
Veratridine
Wine
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Calcium
Catecholamines
Dihydropyridines
Dimethylphenylpiperazinium Iodide
Indoles
NG-Nitroarginine Methyl Ester
Nitric Oxide
Nitric Oxide Synthase
Polyphenols
Receptors, Cholinergic
Sodium
Veratridine
Figure
Cited by 1 articles
-
Quercetin Inhibits
α 3β 4 Nicotinic Acetylcholine Receptor-Mediated Ion Currents Expressed inXenopus Oocytes
Byung-Hwan Lee, Sung-Hee Hwang, Sun-Hye Choi, Tae-Joon Shin, Jiyeon Kang, Sang-Mok Lee, Seung-Yeol Nah
Korean J Physiol Pharmacol. 2011;15(1):17-22. doi: 10.4196/kjpp.2011.15.1.17.
Reference
-
References
Andriambeloson E., Kleschyov AL., Muller B., Beretz A., Stoclet JC., Andriantsitohaina R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br J Pharmacol. 120:1053–1058. 1997.
ArticleAndriambeloson E., Magnier C., Haan-Archipoff G., Lobstein A., Anton R., Beretz A., Stoclet JC., Andriantsitohaina R. Lobstein Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J Nutr. 128:2324–2333. 1998.Andriambeloson E., Stoclet JC., Andriantsitohaina R. Mechanism of endothelial nitric oxide-dependent vasorelaxation induced by wine polyphenols in rat thoracic aorta. J Cardiovasc Pharmacol. 33:248–254. 1999.
ArticleAnton AH., Sayre DF. A study of the factors affecting the aluminum oxide trihydroxy indole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 138:360–375. 1962.Breslow MJ., Tobin JR., Bredt DS., Ferris CD., Snyder SH., Traystman RJ. Nitric oxide as a regulator of adrenal blood flow. Am J Physiol. 264:H464–469. 1993.
ArticleBreslow MJ., Tobin JR., Bredt DS., Ferris CD., Snyder SH., Traystman RJ. Role of nitric oxide in adrenal medullary vasodilation during catecholamine secretion. Eur J Pharmacol. 210:105–106. 1992.
ArticleBurgoyne RD. Mechanism of secretion from adrenal chromaffin cells. Biochem Biophys Acta. 779:201–216. 1984.Caderni G., De Filippo C., Luceri C., Salvadori M., Giannini A., Biggeri A., Remy S., Cheynier V., Dolara P. Effects of black tea, green tea and wine extracts on intestinal carcinogenesis induced by azoxymethane in F344 rats. Carcinogenesis. 21:1965–1969. 2000.
ArticleChalliss RAJ., Jones JA., Owen PJ., Boarder MR. Changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate mass accumulations in cultured adrenal chromaffin cells in response to bradykinin and histamine. J Neurochem. 56:1083–1086. 1991.
ArticleCheek TR., O'Sullivan AJ., Moreton RB., Berridge MJ., Burgoyne RD. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells: Distinct nicotinic and muscarinic patterns. FEBS Lett. 247:429–434. 1989.Chen CK., Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 27:363–366. 1996.Diebolt M., Bucher B., Andriantsitohaina R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension. 38:159–165. 2001.
ArticleFisher SK., Holz RW., Agranoff BW. Muscarinic receptors in chromaffin cell culture mediate enhanced phospholipid labeling but not catecholamine secretion. J Neurochem. 37:491–487. 1981.Fitzpatrick DF., Fleming RC., Bing B., Maggi DA., O'Malley R. Isolation and characterization of endothelium-dependent vasorelaxing compounds from grape seeds. J Agric Food Chem. 48:6384–6390. 2000.
ArticleFlesch M., Schwarz A., Bolun M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am J Physiol. 275:H1183–1190. 1998.
ArticleGarcia AG., Sala F., Reig JA., Viniegra S., Frias J., Fonteriz R., Gandia L. Dihydropyridine Bay-K-8644 activates chromaffin cell calcium channels. Nature. 309:69–71. 1984.
ArticleGoeger DE., Riley RT. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 38:3995–4003. 1989.Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 31:2992–2998. 1982.Ilno M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 94:363–383. 1989.Kidokoro Y., Ritchie AK. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 307:199–216. 1980.
ArticleKilpatrick DL., Slepetis RJ., Corcoran JJ., Kirshner N. Calcium uptake and catecholamine secretion by cultured bovine adrenal medulla cells. J Neurochem. 38:427–435. 1982.
ArticleKilpatrick DL., Slepetis RJ., Kirshner N. Ion channels and membrane potential in stimulus-secretion coupling in adrenal medulla cells. J Neurochem. 36:1245–1255. 1981.
ArticleKnight DE., Kesteven NT. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond Biol Sci. 218:177–199. 1983.Leikert JF., Räthel TR., Wohlfart P., Cheynier V., Vollmar AM., Dirsch VM. Red wine polyphenols enhance endothelial nitric oxide release from endothelial cells. Circulation. 106:1614–1617. 2002.Lim DY. Comparison of green tea extract and epigallocatechin gallate on secretion of catecholamines from the rabbit adrenal medulla. Arch Pharm Res. 28:914–922. 2005.
ArticleLim DY., Hwang DH. Studies on secretion of catecholamines evoked by DMPP and McN-A-343 in the rat adrenal gland. Korean J Pharmacol. 27:53–67. 1991.Lim DY., Kim CD., Ahn KW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 15:115–125. 1992.
ArticleLim DY., Park HG., Lee BR. Green tea extract, not epigallocatechin gallate inhibits catecholamine release from the rat adrenal medulla. J Applied Pharmacol. 11:33–40. 2003.Marley PD., McLeod J., Anderson C., Thomson KA. Nerves containing nitric oxide synthase and their possible function in the control of catecholamine secretion in the bovine adrenal medulla. J Auton Nerv Syst. 54:184–194. 1995.
ArticleMcVeigh GE., Hamilton P., Wilson M., Hanratty CG., Leahey WJ., Devine AB., Morgan DG., Dixon LJ., McGrath LT. Platelet nitric oxide and superoxide release during the development of nitrate tolerance: effect of supplemental ascorbate. Circulation. 106:208–213. 2002.Mizutani K., Ikeda K., Kawai Y., Yamori Y. Extract of wine phenolics improves aortic biomechanical properties in stroke-prone spontaneously hypertensive rats (SHRSP). J Nutr Sci Vitaminol. 45:95–106. 1999.
ArticleNakazato Y., Ohga A., Oleshansky M., Tomita U., Yamada Y. Voltage-independent catecholamine release mediated by the activation of muscarinic receptors in guinea-pig adrenal glands. Br J Pharmacol. 93:101–109. 1988.
ArticleNdiaye M., Chataigneau T., Andriantsitohaina JCS., Schini-Kerth VB. Red wine polyphenols cause endothelium-dependent EDHF-mediated relaxations in porcine coronary arteries via a redox-sensitive mechanism. Biochem Biophys Res Commun. 310:371–377. 2003.
ArticleOka M., Isosaki M., Yanagihara N. Isolated bovine adrenal medullary cells: studies on regulation of catecholamine synthesis and release. Usdin E, Kopin IJ, Brachas J, editors. ed,. Catecholamines: Basic and Clinical frontiers. Pergamon Press;Oxford: p. p. 70–72. 1979.
ArticleOset-Gasque MJ., Parramon M., Hortelano S., Bosca L., Gonzalez MP. Nitric oxide implication in the control of neurosecretion by chromaffin cells. J Neurochem. 63:1693–1700. 1994.
ArticleO'Sullivan AJ., Burgoyne RD. Cyclic GMP regulates nicotine-induced secretion from cultured bovine adrenal chromaffin cells: effects of 8-bromo-cyclic GMP, atrial natriuretic peptide, and nitroprusside (nitric oxide). J Neurochem. 54:1805–1808. 1990.Palacios M., Knowles RG., Palmer RM., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 165:802–809. 1989.
ArticlePalmer RM., Ashton DS., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 333:664–666. 1988.
ArticleRakici O., Kiziltepe U., Coskun B., Aslamaci S., Akar F. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int J Cardiol. 105:209–115. 2005.
ArticleRodriguez-Pascual F., Miras-Portugal MT., Torres M. Effect of cyclic GMP-increasing agents nitric oxide and C-type natriuretic peptide on bovine chromaffin cell function: inhibitory role mediated by cyclic GMP-dependent protein kinase. Mol Pharmacol. 49:1058–1070. 1996.Sakuma I., Stuehr DJ., Gross SS., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 85:8664–8667. 1988.
ArticleSchramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 303:535–537. 1983.Schwarz PM., Rodriguez-Pascual F., Koesling D., Torres M., Förstermann U. Functional coupling of nitric oxide synthase and soluble guanylyl cyclase in controlling catecholamine secretion from bovine chromaffin cells. Neuroscience. 82:255–265. 1998.
ArticleSeidler NW., Jona I., Vegh N., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 264:17816–17823. 1989.Stein JH., Keevil JG., Wiebe DA., Aeschlimann S., Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 100:1050–1055. 1999.
ArticleSuzuki M., Muraki K., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca2+-pump, reduces Ca2+-dependent K+ currents in guinea-pig smooth muscle cells. Br. J Pharmacol. 107:134–140. 1992.Tallarida RJ., Murray RB. Manual of pharmacologic calculation with computer programs. 2nd ed.Speringer-Verlag;New York: p. p. 132. 1987.Torres M., Ceballos G., Rubio R. Possible role of nitric oxide in catecholamine secretion by chromaffin cells in the presence and absence of cultured endothelial cells. J Neurochem. 63:988–996. 1994.
ArticleUchiyama Y., Morita K., Kitayama S., Suemitsu T., Minami N., Miyasako T., Dohi T. Possible involvement of nitric oxide in acetylcholine-induced increase of intracellular Ca2+ concentration and catecholamine release in bovine adrenal chromaffin cells. Jpn J Pharmacol. 65:73–77. 1994.Uyama Y., Imaizumi Y., Watanabe M. Effects of cyclopiazonic acid, a novel Ca2+-ATPase inhibitor on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 106:208–214. 1992.Viveros OH. Mechanism of secretion of catecholaminies from adrenal medulla. In handbook of physiology, Endocrinology. Vol VI, Sect 7, The adrenal gland. American physiological society, Washington DC, p 389–426. 1975.Wada Y., Satoh K., Taira N. Cardiovascular profile of Bay-K-8644, a presumed calcium channel activator in the dog. Naunyn-Schmiedebergs Arch Pharmacol. 328:382–387. 1985a.Wada A., Takara H., Izumi F., Kobayashi H., Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medullary cells. Neuroscience. 15:283–292. 1985b.Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 313:463–480. 1981.
ArticleWakade AR., Wakade TD. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 10:973–978. 1983.
ArticleWallerath T., Deckert G., Ternes T., Anderson H., Li H., Witte K., Förstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 106:1652–1658. 2002.
ArticleZenebe W., Pecháòová O., Andriantsitohaina R. Red wine polyphenols induce vasorelaxation by increased nitric oxide bioactivity. Physiol Res. 52:425–432. 2003.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gintonin facilitates catecholamine secretion from the perfused adrenal medulla
- Inhibitory Effects of Olmesartan on Catecholamine Secretion from the Perfused Rat Adrenal Medulla
- Influence of SKF81297 on Catecholamine Release from the Perfused Rat Adrenal Medulla
- Mechanism of Leptin-Induced Potentiation of Catecholamine Secretion Evoked by Cholinergic Stimulation in the Rat Adrenal Medulla
- Comparison of Inhibitory Effects between Enalapril and Losartan on Adrenal Catecholamine Secretion