Korean J Physiol Pharmacol.
2008 Dec;12(6):323-330. 10.4196/kjpp.2008.12.6.323.
Voltage-dependent Ca2+ Current Identified in Freshly Isolated Interstitial Cells of Cajal (ICC) of Guinea-pig Stomach
- Affiliations
-
- 1Department of Physiology, Chungbuk National University, College of Medicine, Cheongju 361-763, Korea.
- 2Department of Physiology, Nagoya City University Medical School, Nagoya 467-8601, Japan.
- 3Department of Physiology, College of Medicine, Shanghai Jiaotong University, Shanghai 200240, P.R. China.
- 4Department of Pharmacology, Chungbuk National University, College of Medicine, Cheongju 361-763, Korea.
- 5Department of Surgery, Chungbuk National University, College of Medicine, Cheongju 361-763, Korea.
- 6Department of Internal Medicine, Chungbuk National University, College of Medicine, Cheongju 361-763, Korea.
- KMID: 2285359
- DOI: http://doi.org/10.4196/kjpp.2008.12.6.323
Abstract
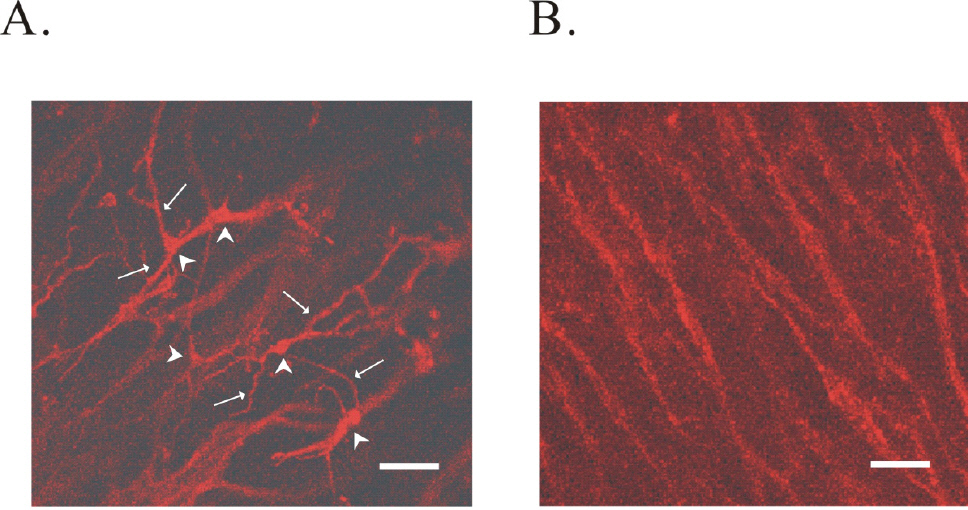
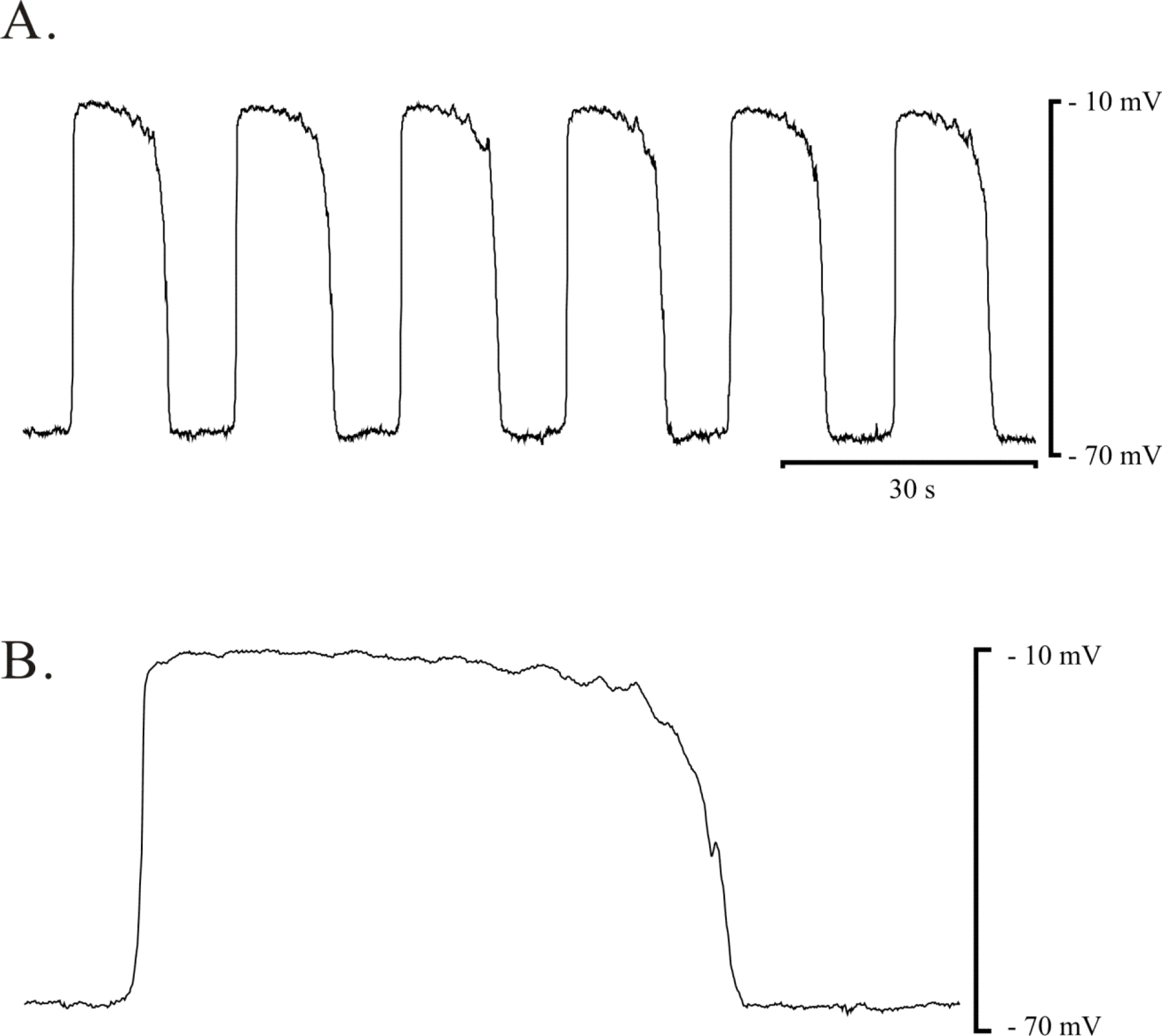
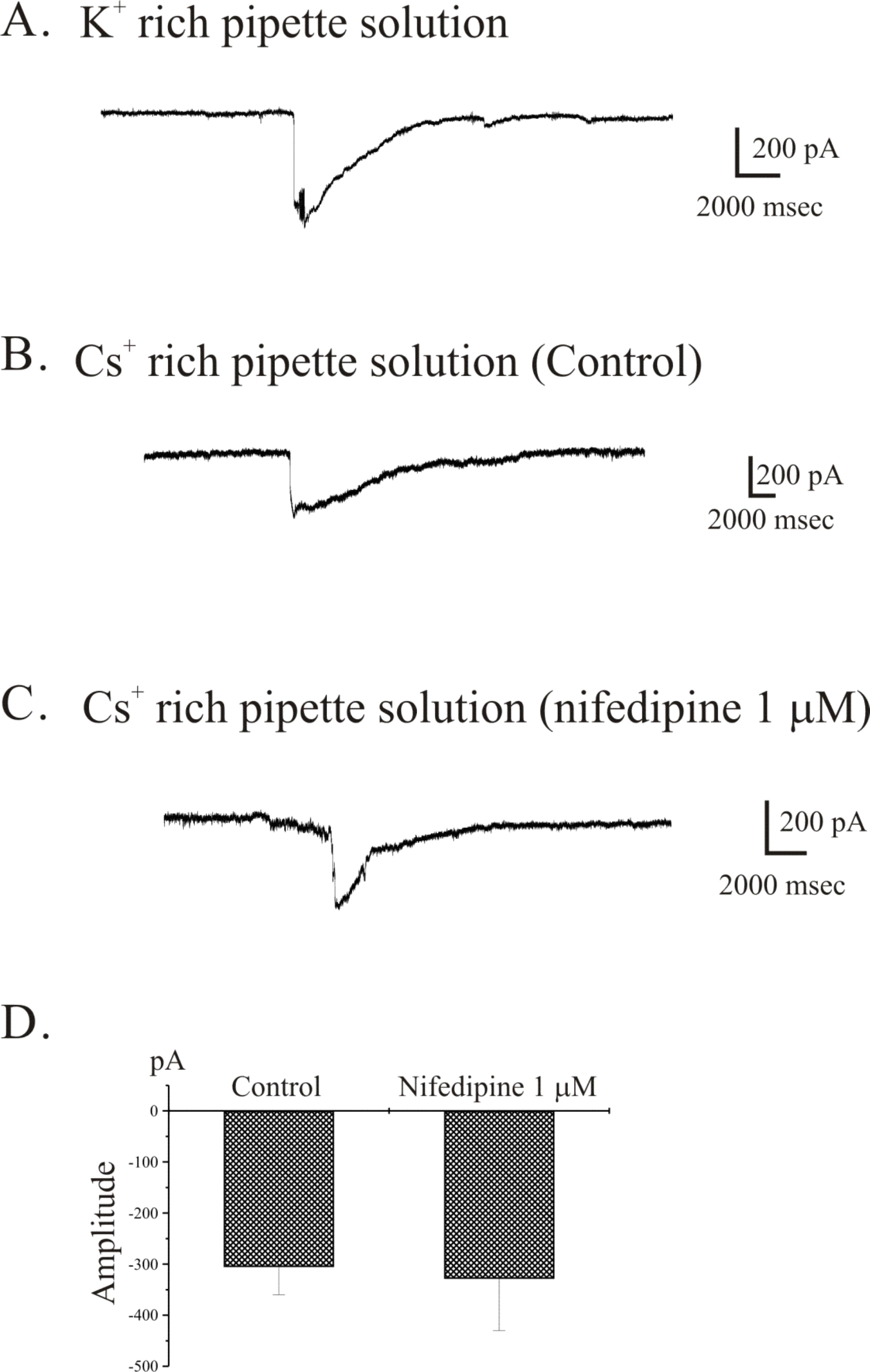
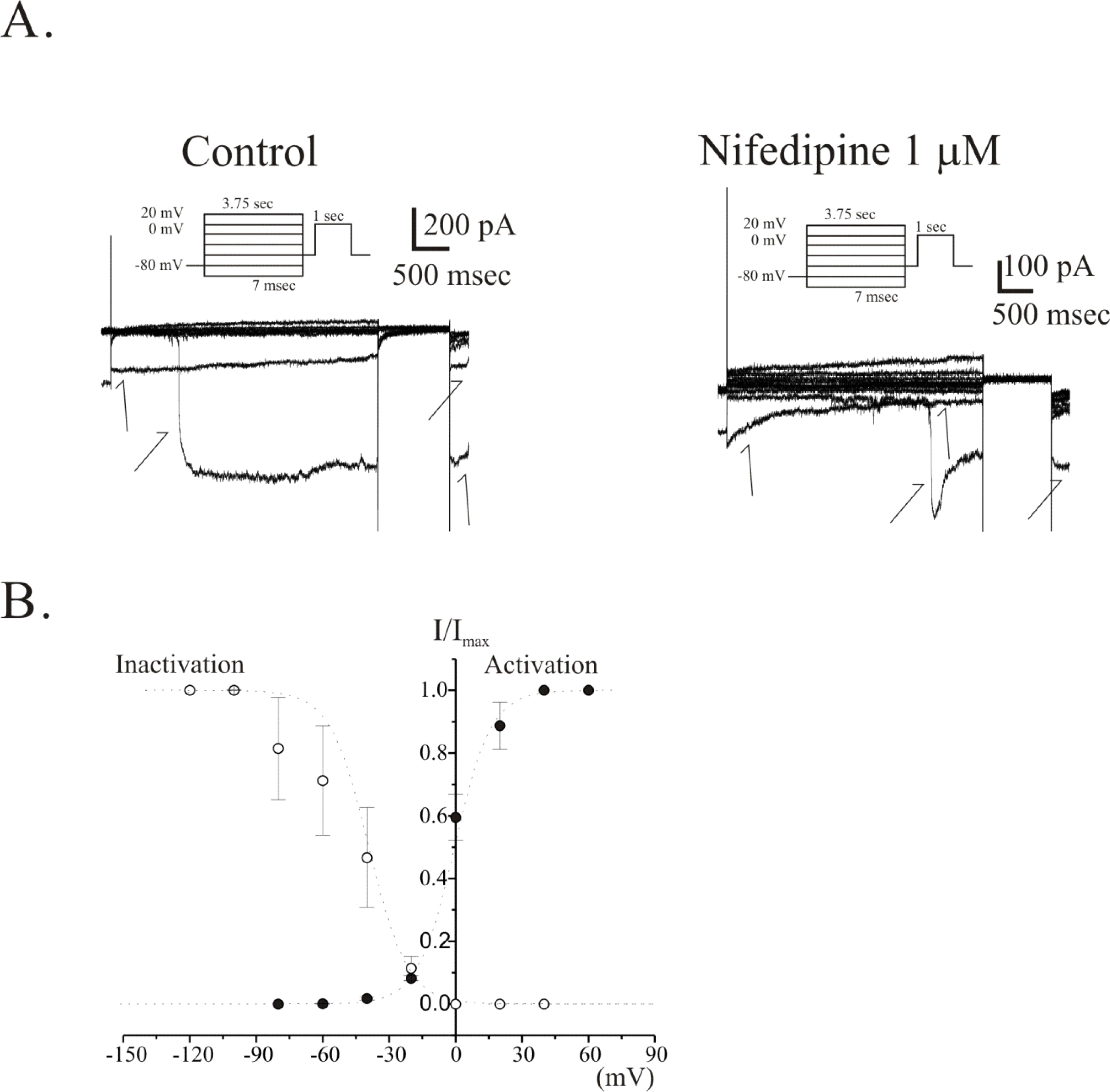
- The properties of voltage dependent Ca2+ current (VDCC) were investigated in interstitial cells of Cajal (ICC) distributed in the myenteric layer (ICC-MY) of guinea-pig antrum. In tissue, ICC-MY showed c-Kit positive reactions and produced driving potentials with the amplitude and frequency of about 62 mV and 2 times min(-1), respectively, in the presence of 1micrometer nifedipine. Single ICC-MY isolated by enzyme treatment also showed c-Kit immunohistochemical reactivity. These cells were also identified by generation of spontaneous inward current under K+-rich pipette solution. The voltage clamp experiments revealed the amplitude of - 329 pA inward current at irregular frequency. With Cs+-rich pipette solution at Vh=?80 mV, ICC-MY produced voltage-dependent inward currents (VDIC), and nifedipine (1micrometer) blocked VDIC. Therefore, we successfully isolated c-Kit positive single ICC from guinea-pig stomach, and found that ICC-MY potently produced dihydropiridine sensitive L-type voltage-dependent Ca2+ currents (VDCCL).
Keyword
Figure
Reference
-
Beckett EAH., Ro S., Bayquinov Y., Sanders KM., Ward SM. Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic and gastrointestinal tract. Dev Dyn. 236:60–72. 2007.Cayabyab FS., DeBruin H., Jimenez M., Daniel EE. Ca2+ role in myogenic and neurogenic activities of canine ileum circular muscle. Am J Physiol. 271:G1053–G1066. 1996.Dickens EJ., Edward FR., Hirst GDS. Selective knockout of intramuscular interstitial cell reveals their role in the generation of slow waves in mouse stomach. J Physiol. 531:827–833. 2001.Goto K., Matsuoka S., Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol. 559:409–420. 2004.
ArticleDickens EJ., Hirst GDS., Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 514:515–531. 1999.
ArticleHamil OP., Marty A., Neher E., Sakamnn B., Sigworth FJ. Improved patch-clamp technique for high resolution current from cells ands cell-free membrane patches. Pflügers Arch. 391:85–100. 1981.Hennig GW., Hirst GD., Park KJ., Smith CB., Sanders KM., Ward SM., Smith TK. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol. 556:585–599. 2004.
ArticleHirst GD., Edward FR. Generation of slow waves in the antral region of guinea-pig stomach – a stochastic process. J Physiol. 535:165–180. 2001.
ArticleHirst GDS., Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 550:337–346. 2003.
ArticleHuizinga JD., Thuneberg L., Kluppel M., Malysz J., Mikkelsen HB., Bernstein A. W/Kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 373:347–349. 1995.Kim YC., Koh SD., Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 541:797–810. 2002.Kito Y., Suzuki H. Pacemaker frequency is increased by sodium nitroprusside in the guinea pig gastric antrum. J Physiol. 546:191–205. 2003.
ArticleKito Y., Ward SM., Sanders KM. Pacemaker potential generated by interstitial cells of Cajal in the murine intestine. J Physiol. 288:C710–C720. 2005.Klüppel M., Huizinga JD., Malysz J., Bernstein A. Developmental origin and Kit- dependent development of the interstitial cells of Cajal in the mammalian small intestine. Dev Dyn. 211:60–71. 1998.Koh SD., Jun JY., Kim TW., Sanders KM. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cells of Cajal. J Physiol. 540:803–814. 2002.Koh SD., Monaghhan K., Ro S., Mason HS., Kenyon JL., Sanders KM. Novel voltage-dependent non-selective cation conductance in murine colonic myocytes. J Physiol. 533:341–355. 2001.
ArticleKoh SD., Sanders KM., Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 513:203–213. 1998.
ArticleKomuro T., Tokui K., Zhou DS. Identification of interstitial cells of Cajal. Histol Histopathol. 11:769–786. 1996.Kubota M., Kanda E., Ida K., Sakakihara Y., Hayashi M. Severe gastrointestinal dysmotility in a patient with congenital myopathy: causal relationship to decrease of interstitial cells of Cajal. Brain Develop. 27:447–450. 2005.
ArticleLammers WJ., Stephen B., Adeghate E., Ponery S., Pozzan O. The slow wave does not propagate across the gastroduodenal junction in the isolated feline preparation. Neurogastroenterol Motil. 10:339–349. 1998.
ArticleLangton P., Ward SM., Carl A., Norell MA., Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci. 86:7280–7284. 1989.
ArticleLee HK., Sanders KM. Comparision of ionic currents from interstitial cells and smooth muscle cells of canine colon. J Physiol. 460:135–152. 1993.Lee JI., Park HJ., Kamm MA., Talbot IC. Decreased density of interstitial cells of Cajal and neuronal cells in patients with slow-transit constipation and acquired megacolon. J Gastroenterol Hepatol. 20:1292–1298. 2005.
ArticleMalysz J., Richaedson D., Farraway L., Christen MOM., Huizinga JD. Generaion of slow wave type action potentials in the mouse small intestine involves a non-L-type calsium channel. Can J Physiol Pharmacol. 73:1502–1511. 1995.Ördög T., Redelman D., Miller LJ., Horváth VJ., Zhong Q., Almeida-Porada G., Zanjani ED., Horowitz B., Sanders KM. Purification of interstitial cells of Cajal by fluorescence-activated cell sorting. Am J Physiol. 286:C448–C456. 2004.
ArticleOue T., Puri P. Smooth muscle cell hypertrophy versus hyperplasia in infantile hypertrophic pyloric stenosis. Pediatric Res. 45:853–857. 1999.
ArticlePucovsky V., Moss Ray., Bolton TB. Non-contractile cells with thin processes resembling interstitial cells of Cajal found in the wall of guinea-pig mesenteric arteries. J Physiol. 552:119–133. 2003.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 111:492–515. 1996.
ArticleSergeant GP., Hollywood MA., McCloskey KD., Thornbury KD., McHale NG. Specialized pacemaking cells in the rabbit urethra. J Physiol. 526:359–366. 2000.Thomsen L., Robinson TL., Lee JCF., Farraway LA., Hughes MJG., Andrew DW., Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 4:848–851. 1998.
ArticleTorihashi S., Ward SM., Nishikawa S., Kobayashi S., Sanders KM. c-Kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 280:97–111. 1995.Torihashi S., Ward SM., Sanders KM. Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 112:144–155. 1997.Wang B., Kunze WA., Zhu Y., Huizinga JD. In situ recording from gut pacemaker cells. Pflügers Arch. 457:243–251. 2008.
ArticleWang XY., Lammers WJEP., Bercik P., Huizinga JD. Lack of pyloric interstitial cells of Cajal explains distinct peristalsis motor patterns in stomach and small intestine. Am J Physiol. 289:G539–G549. 2005.Wang XY., Paterson C., Huizinga JD. Cholineregic and nitrergic innervation of ICC-DMP and ICC-IM in the human small intestine. Neurogastroenterol Motil. 15:531–543. 2003.Ward SM., Burns AJ., Torihashi S., Sanders KM. Mutation of the proto-oncogene c-Kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 480:91–97. 1994.Ward SM., Dixon RE., Faoite A., Sanders KM. Voltage-dependent calcium entry underlies propagation of slow waves in canine gastric antrum. J Physiol. 561:793–810. 2004.
ArticleWard SM., Sanders KM. Upstroke potential of electrical slow waves in canine colonic smooth muscle due to nifedipine-resistant calcium current. J Physiol. 455:321–337. 1992.Xu WX., Kim SJ., Kim SJ., So I., Kang TM., Rhee JC., Kim KW. Effect of stretch on calcium channel currents recorded from the antral myocytes of guinea-pig stomach. Pflügers Arch. 432:159–164. 1996.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ca2+-activated K+ Current in Freshly Isolated c-Kit Positive Cells in Guinea-pig Stomach
- Depolarizing Mechanisms of Acetylcholine in Gastric Smooth Muscle
- The Effect of External Divalent Cations on Intestinal Pacemaking Activity
- Capsaicin Inhibits the Spontaneous Pacemaker Activity in Interstitial Cells of Cajal From the Small Intestine of Mouse
- Properties of spontaneous activity in gastric smooth muscle