Infect Chemother.
2013 Jun;45(2):159-174. 10.3947/ic.2013.45.2.159.
Fluad(R)-MF59(R)-Adjuvanted Influenza Vaccine in Older Adults
- Affiliations
-
- 1Novartis Vaccines and Diagnostics Inc., 350 Massachusetts Ave, Cambridge, USA. theodore.tsai@novartis.com
- KMID: 2284997
- DOI: http://doi.org/10.3947/ic.2013.45.2.159
Abstract
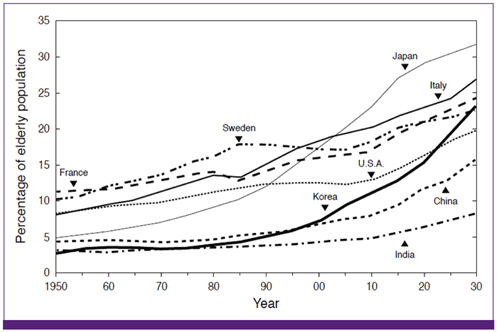
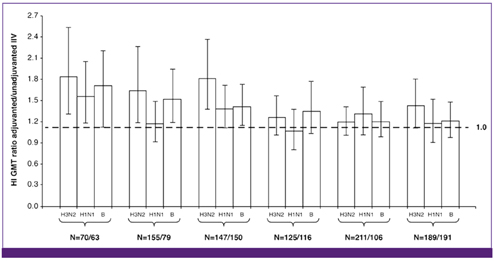
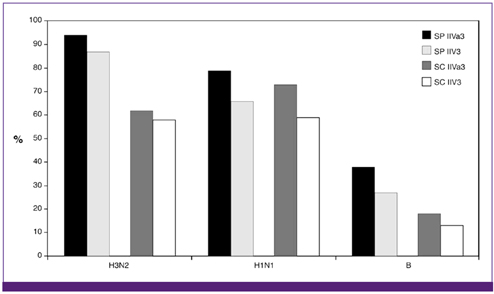
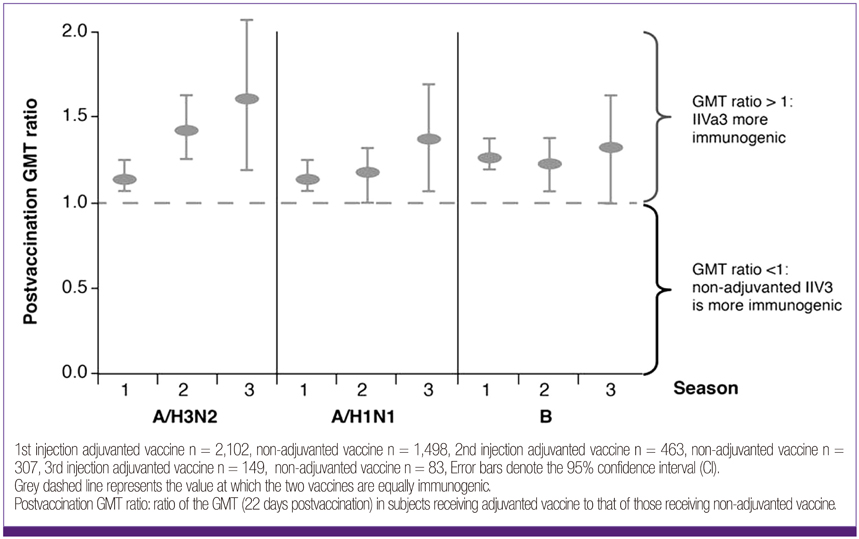
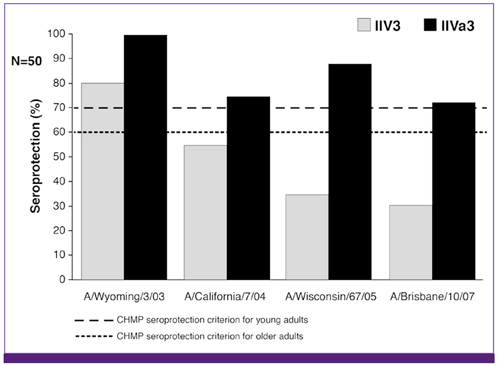
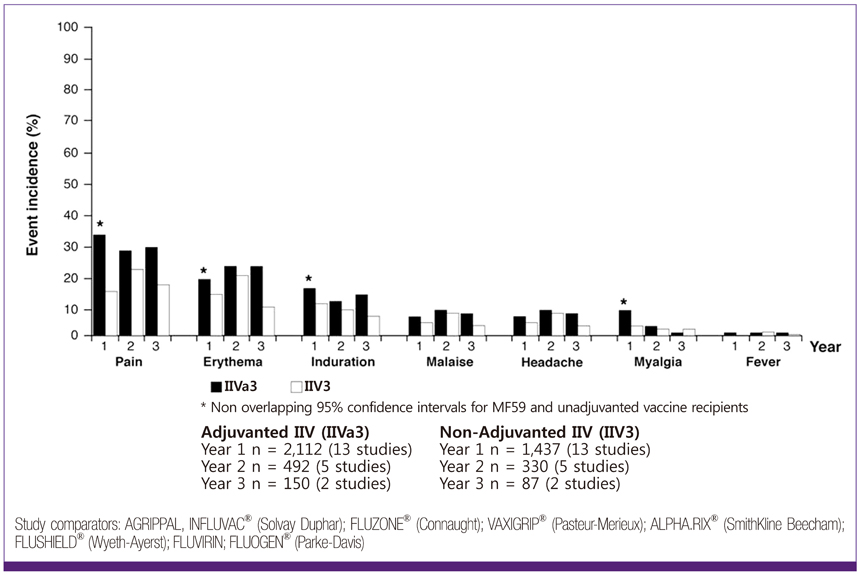
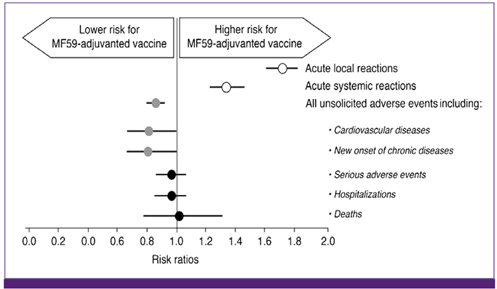
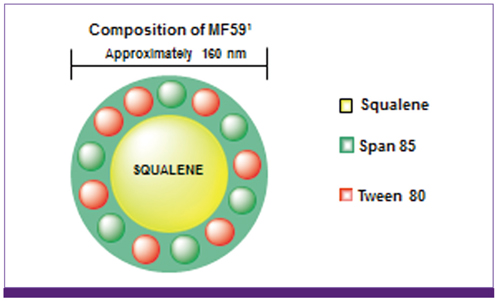
- Influenza directly or indirectly contributes to the four leading causes of global mortality, at rates that are highest in older adults. As the proportion of older adults in the Korean population is greater than in most other countries, influenza prevention is a greater public health priority in Korea than elsewhere. Conventional inactivated influenza vaccine (IIV) is less immunogenic and efficacious (-50%) in older than in young adults, but adjuvanting the vaccine with oil-in-water emulsion MF59(R) increases immunogenicity, resulting in comparatively higher levels of hemagglutination inhibition antibodies and greater protection against all influenza, as well as cases requiring hospitalization. A recent observational study demonstrated that the adjuvanted vaccine protected older adults against influenza in a year when nonadjuvanted IIV was ineffective. In another multiyear study, the adjuvanted vaccine was estimated to be 25% more effective in preventing pneumonia and influenza hospitalizations compared to nonadjuvanted vaccine. Although MF59-adjuvanted vaccine is transiently more reactogenic than nonadjuvanted vaccine, there is no evidence that it increases risks for serious adverse events, including those with an autoimmune etiology. Experience thus far indicates a favorable balance of benefit to risk for MF59. This may reflect the adjuvant's mechanism of action in which the squalene oil emulsion increases antibody responses to co-administered antigen without acting more generally as an immunopotentiator.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Influenza Vaccines: Unmet Needs and Recent Developments
Ji Yun Noh, Woo Joo Kim
Infect Chemother. 2013;45(4):375-386. doi: 10.3947/ic.2013.45.4.375.Phase 4, Post-Marketing Safety Surveillance of the MF59-Adjuvanted Influenza Vaccines FLUAD® and VANTAFLU® in South Korean Subjects Aged ≥65 Years
Byung Wook Yoo, Chang Oh Kim, Allen Izu, Ashwani Kumar Arora, Esther Heijnen
Infect Chemother. 2018;50(4):301-310. doi: 10.3947/ic.2018.50.4.301.
Reference
-
1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012. 380:2095–2128.
Article2. Monto AS, Ansaldi F, Aspinall R, McElhaney JE, Montaño LF, Nichol KL, Puig-Barberà J, Schmitt J, Stephenson I. Influenza control in the 21st century: Optimizing protection of older adults. Vaccine. 2009. 27:5043–5053.
Article3. Statistics Bureau. Statistical Handbook of Japan 2012. Accessed 1 November 2012. Available at: http://www.stat.go.jp/english/data/handbook/.4. Cai L, Uchiyama H, Yanagisawa S, Kamae I. Cost-effectiveness analysis of influenza and pneumococcal vaccinations among elderly people in Japan. Kobe J Med Sci. 2006. 52:97–109.5. Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012. 12:36–44.
Article6. Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007. 7:658–666.
Article7. Thijs C, Beyer WE, Govaert PM, Sprenger MJ, Dinant GJ, Knottnerus A. Mortality benefits of influenza vaccination in elderly people. Lancet Infect Dis. 2008. 8:460–461. author reply 463-5.
Article8. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006. 24:1159–1169.
Article9. Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, Cho GJ, Hwang TG, Kim WJ. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010. 28:3929–3935.
Article10. Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, Sambhara S. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging Dis. 2012. 3:68–90.11. Orsi A, Ansaldi F, de Florentiis D, Ceravolo A, Parodi V, Canepa P, Coppelli M, Icardi G, Durando P. Cross-protection against drifted influenza viruses: Options offered by adjuvanted and intradermal vaccines. Hum Vaccin Immunother. 2013. 9:[Epub ahead of print].12. Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine. 2001. 19:2673–2680.
Article13. O'Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of MF59(®) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines. 2013. 12:13–30.14. Vancouver Coastal Health. Physicians' Update - 1 October 2012. Accessed 10 October 2012. Available at: http://www.vch.ca/your_health/health_topics/communicable_diseases/for_health_professionals/physician_updates/physicians__update/.15. Ansaldi F, Bacilieri S, Durando P, Sticchi L, Valle L, Montomoli E, Icardi G, Gasparini R, Crovari P. Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine. 2008. 26:1525–1529.
Article16. Squarcione S, Sgricia S, Biasio LR, Perinetti E. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine. 2003. 21:1268–1274.
Article17. Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, Monto AS. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013. 56:1363–1369.
Article18. Song JY, Cheong HJ, Seo YB, Kim IS, Noh JY, Choi WS, Lee J, Jeong HW, Kee SY, Kim WJ. Long-term immunogenicity of the pandemic influenza A/H1N1 2009 vaccine among health care workers: influence of prior seasonal influenza vaccination. Clin Vaccine Immunol. 2013. 20:513–516.
Article19. Medina RA, García-Sastre A. Influenza A viruses: new research developments. Nat Rev Microbiol. 2011. 9:590–603.
Article20. Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, Gust ID, Hampson AW, Hay AJ, Hurt AC, de Jong JC, Kelso A, Klimov AI, Kageyama T, Komadina N, Lapedes AS, Lin YP, Mosterin A, Obuchi M, Odagiri T, Osterhaus AD, Rimmelzwaan GF, Shaw MW, Skepner E, Stohr K, Tashiro M, Fouchier RA, Smith DJ. The global circulation of seasonal influenza A (H3N2) viruses. Science. 2008. 320:340–346.
Article21. Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011. 3:85ra48.
Article22. Kang S, Yang IS, Lee JY, Park Y, Oh HB, Kang C, Kim KH. Epidemiologic study of human influenza virus infection in South Korea from 1999 to 2007: origin and evolution of A/Fujian/411/2002-like strains. J Clin Microbiol. 2010. 48:2177–2185.
Article23. Cheng X, Tan Y, He M, Lam TT, Lu X, Viboud C, He J, Zhang S, Lu J, Wu C, Fang S, Wang X, Xie X, Ma H, Nelson MI, Kung HF, Holmes EC, Cheng J. Epidemiological dynamics and phylogeography of influenza virus in southern China. J Infect Dis. 2013. 207:106–114.
Article24. Centers for Disease Control and Prevention. FluView: 2012-2013 Influenza season week 13 ending March 30, 2013. Accessed 2 April 2013. Available at: http://www.cdc.gov/flu/weekly/.25. Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012. 54:1427–1436.
Article26. Madjid M, Awan I, Ali M, Frazier L, Casscells W. Influenza and atherosclerosis: vaccination for cardiovascular disease prevention. Expert Opin Biol Ther. 2005. 5:91–96.
Article27. Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med. 1998. 158:645–650.
Article28. Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, Burant C, Covinsky KE. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008. 56:2171–2179.
Article29. Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, Burant CJ, Landefeld CS. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003. 51:451–458.
Article30. Seo YB, Hong KW, Kim IS, Choi WS, Baek JH, Lee J, Song JY, Lee JS, Cheong HJ, Kim WJ. Effectiveness of the influenza vaccine at preventing hospitalization due to acute lower respiratory infection and exacerbation of chronic cardiopulmonary disease in Korea during 2010-2011. Vaccine. 2013. 31:1426–1430.
Article31. Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011-2012 influenza season. Clin Infect Dis. 2013. 56:1774–1777.
Article32. Puig-Barberà J, Diez-Domingo J, Pérez Hoyos S, Belenguer Varea A, González Vidal D. Effectiveness of the MF59-adjuvanted influenza vaccine in preventing emergency admissions for pneumonia in the elderly over 64 years of age. Vaccine. 2004. 23:283–289.
Article33. Puig-Barberà J, Díez-Domingo J, Varea AB, Chavarri GS, Rodrigo JA, Hoyos SP, Vidal DG. Effectiveness of MF59-adjuvanted subunit influenza vaccine in preventing hospitalisations for cardiovascular disease, cerebrovascular disease and pneumonia in the elder ly. Vaccine. 2007. 25:7313–7321.
Article34. Gasparini R, Amicizia D, Lai PL, Rossi S, Panatto D. Effectiveness of adjuvanted seasonal influenza vaccines (Inflexal V® and Fluad®) in preventing hospitalization for influenza and pneumonia in the elderly: a matched case-control study. Hum Vaccin Immunother. 2013. 9:144–152.
Article35. Puig-Barberà J, Díez-Domingo J, Arnedo-Pena A, Ruiz-García M, Pérez-Vilar S, Micó-Esparza JL, Belenguer-Varea A, Carratalá-Munuera C, Gil-Guillén V, Schwarz-Chavarri H. Effectiveness of the 2010-2011 seasonal influenza vaccine in preventing confirmed influenza hospitalizations in adults: a case-case comparison, case-control study. Vaccine. 2012. 30:5714–5720.
Article36. Eick-Cost AA, Tastad KJ, Guerrero AC, Johns MC, Lee SE, Macintosh VH, Burke RL, Blazes DL, Russell KL, Sanchez JL. Effectiveness of seasonal influenza vaccines against influenza-associated illnesses among US military personnel in 2010-11: a case-cont rol approach. PLoS One. 2012. 7:e41435.37. Kissling E, Valenciano M, Cohen JM, Oroszi B, Barret AS, Rizzo C, Stefanoff P, Nunes B, Pitigoi D, Larrauri A, Daviaud I, Horvath JK, O'Donnell J, Seyler T, Paradowska-Stankiewicz IA, Pechirra P, Ivanciuc AE, Jiménez-Jorge S, Savulescu C, Ciancio BC, Moren A. I-MOVE multi-centre case control study 2010-11: overall and stratified estimates of influenza vaccine effectiveness in Europe. PLoS One. 2011. 6:e27622.
Article38. Pebody RG, Andrews N, Fleming DM, McMenamin J, Cottrell S, Smyth B, Durnall H, Robertson C, Carman W, Ellis J, Sebastian-Pillai P, Zambon M, Kearns C, Moore C, Thomas DR, Watson JM. Age-specific vaccine effectiveness of seasonal 2010/2011 and pandemic influenza A(H1N1) 2009 vaccines in preventing influenza in the United Kingdom. Epidemiol Infect. 2012. 1–11.
Article39. Skowronski DM, Janjua NZ, De Serres G, Winter AL, Dickinson JA, Gardy JL, Gubbay J, Fonseca K, Charest H, Crowcroft NS, Fradet MD, Bastien N, Li Y, Krajden M, Sabaiduc S, Petric M. A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010-2011 season. Clin Infect Dis. 2012. 55:332–342.
Article40. Treanor JJ, Talbot HK, Ohmit SE, Coleman LA, Thompson MG, Cheng PY, Petrie JG, Lofthus G, Meece JK, Williams JV, Berman L, Breese Hall C, Monto AS, Griffin MR, Belongia E, Shay DK. US Flu-VE Network. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis. 2012. 55:951–959.
Article41. Iob A, Brianti G, Zamparo E, Gallo T. Evidence of increased clinical protection of an MF59-adjuvant influenza vaccine compared to a non-adjuvant vaccine among elderly residents of long-term care facilities in Italy. Epidemiol Infect. 2005. 133:687–693.
Article42. Mannino S, Villa M, Apolone G, Weiss NS, Groth N, Aquino I, Boldori L, Caramaschi F, Gattinoni A, Malchiodi G, Rothman KJ. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol. 2012. 176:527–533.
Article43. Levandowski RA, Regnery HL, Staton E, Burgess BG, Williams MS, Groothuis JR. Antibody responses to influenza B viruses in immunologically unprimed children. Pediatrics. 1991. 88:1031–1036.
Article44. Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012. 30:1993–1998.
Article45. Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, van der Vlugt R, Lamrani M, Korse HJ, Geelen E, Sahin Ö, Sieuwerts M, Brakenhoff JP, Vogels R, Li OT, Poon LL, Peiris M, Koudstaal W, Ward AB, Wilson IA, Goudsmit J, Friesen RH. Highly conserved protective epitopes on influenza B viruses. Science. 2012. 337:1343–1348.
Article46. Janjua NZ, Skowronski DM, De Serres G, Dickinson J, Crowcroft NS, Taylor M, Winter AL, Hottes TS, Fonseca K, Charest H, Drews SJ, Sabaiduc S, Bastien N, Li Y, Gardy JL, Petric M. Estimates of influenza vaccine effectiveness for 2007-2008 from Canada's sentinel surveillance system: cross-protection against major and minor variants. J Infect Dis. 2012. 205:1858–1868.
Article47. Konrad S. Influenza Comparative Vaccine Effectiveness Study. In : Annual meeting of 10th Canadian Immunization Conference; 2012 Dec 4; Vancouver, Canada.48. Castilla J, Martínez-Baz I, Martínez-Artola V, Reina G, Pozo F, García Cenoz M, Guevara M, Morán J, Irisarri F, Arriazu M, Albéniz E, Ezpeleta C, Barricarte A. Primary Health Care Sentinel Network. Network for Influenza Surveillance in Hospitals of Navarre. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013. 18:pii: 20388.
Article49. Kissling E, Valenciano M, Larrauri A, Oroszi B, Cohen JM, Nunes B, Pitigoi D, Rizzo C, Rebolledo J, Paradowska-Stankiewicz I, Jiménez-Jorge S, Horváth JK, Daviaud I, Guiomar R, Necula G, Bella A, O'Donnell J, Głuchowska M, Ciancio BC, Nicoll A, Moren A. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Euro Surveill. 2013. 18:pii: 20390.
Article50. MacIntosh VH, Tastad KJ, Eick-Cost AA. Mid-season influenza vaccine effectiveness 2011-2012: a Department of Defense Global, Laboratory-based, Influenza Surveillance System case-control study estimate. Vaccine. 2013. 31:1651–1655.
Article51. Pebody R, Andrews N, McMenamin J, Durnall H, Ellis J, Thompson CI, Robertson C, Cottrell S, Smyth B, Zambon M, Moore C, Fleming DM, Watson JM. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: evidence of waning intra-seasonal protection. Euro Surveill. 2013. 18:pii: 20389.
Article52. Schultze V, D'Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008. 26:3209–3222.
Article53. Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine. 2009. 27:6959–6965.
Article54. Cheong HJ, Song JY, Heo JY, Noh JY, Choi WS, Park DW, Wie SH, Kim WJ. Immunogenicity and safety of the influenza A/H1N1 2009 inactivated split-virus vaccine in young and older adults: MF59-adjuvanted vaccine versus nonadjuvanted vaccine. Clin Vaccine Immunol. 2011. 18:1358–1364.
Article55. Dell'Era L, Corona F, Daleno C, Scala A, Principi N, Esposito S. Immunogenicity, safety and tolerability of MF59-adjuvanted seasonal influenza vaccine in children with juvenile idiopathic arthritis. Vaccine. 2012. 30:936–940.56. Moro ML, Nobilio L, Voci C, Di Mario S, Candela S, Magrini N. SaFoH1N1 working group. A population based cohort study to assess the safety of pandemic influenza vaccine Focetria in Emilia-Romagna region, Italy - part two. Vaccine. 2013. 31:1438–1446.
Article57. Candela S, Pergolizzi S, Ragni P, Cavuto S, Nobilio L, Di Mario S, Dragosevic V, Groth N, Magrini N. SaFoH1N1 working group. An early (3-6 weeks) active surveillance study to assess the safety of pandemic influenza vaccine Focetria® in a province of Emilia-Romagna region, Italy - part one. Vaccine. 2013. 31:1431–1437.
Article58. Isai A, Durand J, Le Meur S, Hidalgo-Simon A, Kurz X. Autoimmune disorders after immunisation with Influenza A/H1N1 vaccines with and without adjuvant: EudraVigilance data and literature review. Vaccine. 2012. 30:7123–7129.
Article59. Banzhoff A, Haertel S, Praus M. Passive surveillance of adverse events of an MF59-adjuvanted H1N1v vaccine during the pandemic mass vaccinations. Hum Vaccin. 2011. 7:539–548.
Article60. Moro ML, Nobilio L, Voci C, Di Mario S, Candela S, Magrini N. SaFoH1N1 working group. A population based cohort study to assess the safety of pandemic influenza vaccine Focetria in Emilia-Romagna region, Italy - part two. Vaccine. 2013. 31:1438–1446.
Article61. Biscayart C. Argentina's integral prevention of influenza: a successful experience of an emerging country. In : Annual meeting of Influenza vaccines for the world; 2012 Oct 10; Valencia, Spain.62. Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsén P, Saarenpää-Heikkilä O, Kilpi T. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012. 7:e33536.
Article63. Miller E, Andrews N, Stellitano L, Stowe J, Winstone AM, Shneerson J, Verity C. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ. 2013. 346:f794.
Article64. Tsai TF, Crucitti A, Nacci P, Nicolay U, Della Cioppa G, Ferguson J, Clemens R. Explorations of clinical trials and pharmacovigilance databases of MF59®-adjuvanted influenza vaccines for associated cases of narcolepsy. Scand J Infect Dis. 2011. 43:702–706.
Article65. Tsai TF, Del Giudice G, Crucitti A, Weil J, Narasimhan V. Is the adjuvant solely to blame? BMJ. 2013. 346:f2375.
Article66. Choe YJ, Bae GR, Lee DH. No association between influenza A(H1N1)pdm09 vaccination and narcolepsy in South Korea: an ecological study. Vaccine. 2012. 30:7439–7442.
Article67. Han F, Lin L, Li J, Dong XS, Mignot E. Decreased incidence of childhood narcolepsy 2 years after the 2009 H1N1 winter flu pandemic. Ann Neurol. 2012. [Epub ahead of print].
Article68. Han F, Lin L, Warby SC, Faraco J, Li J, Dong SX, An P, Zhao L, Wang LH, Li QY, Yan H, Gao ZC, Yuan Y, Strohl KP, Mignot E. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011. 70:410–417.
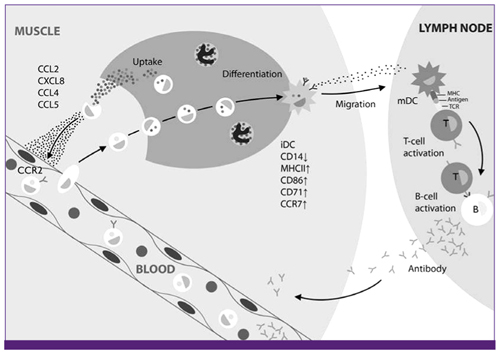
Article69. O'Hagan DT, Ott GS, De Gregorio E, Seubert A. The mechanism of action of MF59 - an innately attractive adjuvant formulation. Vaccine. 2012. 30:4341–4348.70. Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O'Hagan DT, De Gregorio E, Seubert A, Wack A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011. 29:1812–1823.
Article71. Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O'Hagan D, Rappuoli R, De Gregorio E. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A. 2008. 105:10501–10506.
Article72. Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, Aprea S, Colaprico A, D'Oro U, Giuliani MM, Pallaoro M, Pizza M, O'Hagan DT, Wack A, Rappuoli R, De Gregorio E. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A. 2011. 108:11169–11174.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phase 4, Post-Marketing Safety Surveillance of the MF59-Adjuvanted Influenza Vaccines FLUAD® and VANTAFLU® in South Korean Subjects Aged ≥65 Years
- The Korean Influenza National Immunization Program: History and Present Status
- Influenza Vaccine
- Seasonal influenza and vaccine herd effect
- Assessment of mOMV adjuvant efficacy in the pathogenic H1N1 influenza virus vaccine