Diabetes Metab J.
2012 Feb;36(1):29-36. 10.4093/dmj.2012.36.1.29.
Various Oscillation Patterns of Serum Fibroblast Growth Factor 21 Concentrations in Healthy Volunteers
- Affiliations
-
- 1Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea.
- 2Department of Internal Medicine, Seoul Veterans Hospital, Seoul, Korea.
- 3Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. mskim@amc.seoul.kr
- KMID: 2281426
- DOI: http://doi.org/10.4093/dmj.2012.36.1.29
Abstract
- BACKGROUND
Fibroblast growth factor 21 (FGF21) was originally identified as a paroxysm proliferator activated receptor-alpha target gene product and is a hormone involved in metabolic regulation. The purpose of this study was to investigate the diurnal variation of serum FGF21 concentration in obese and non-obese healthy volunteers.
METHODS
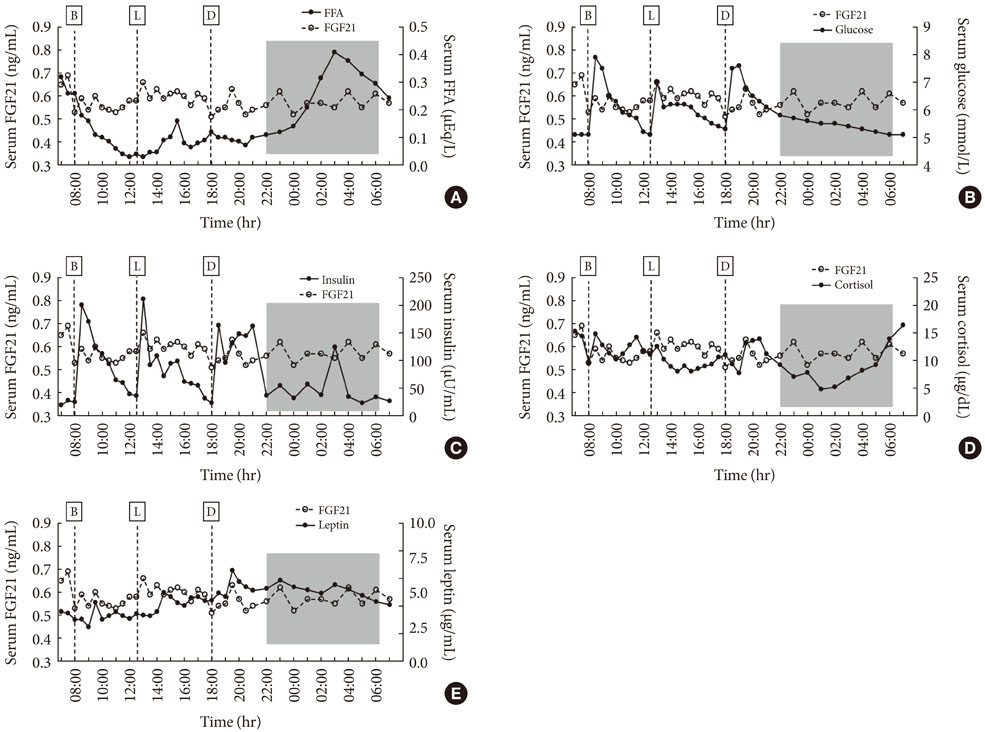
Blood samples were collected from five non-obese (body mass index [BMI] < or =23 kg/m2) and five obese (BMI > or =25 kg/m2) healthy young men every 30 to 60 minutes over 24 hours. Serum FGF21 concentrations were determined by radioimmunoassay. Anthropometric parameters, glucose, free fatty acid, insulin, leptin, and cortisol concentrations were also measured.
RESULTS
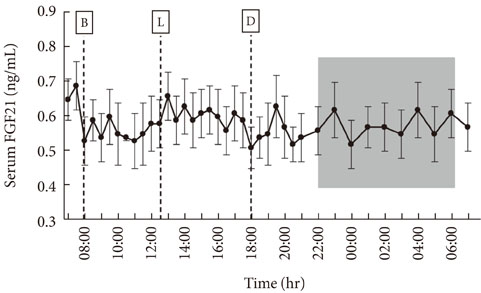
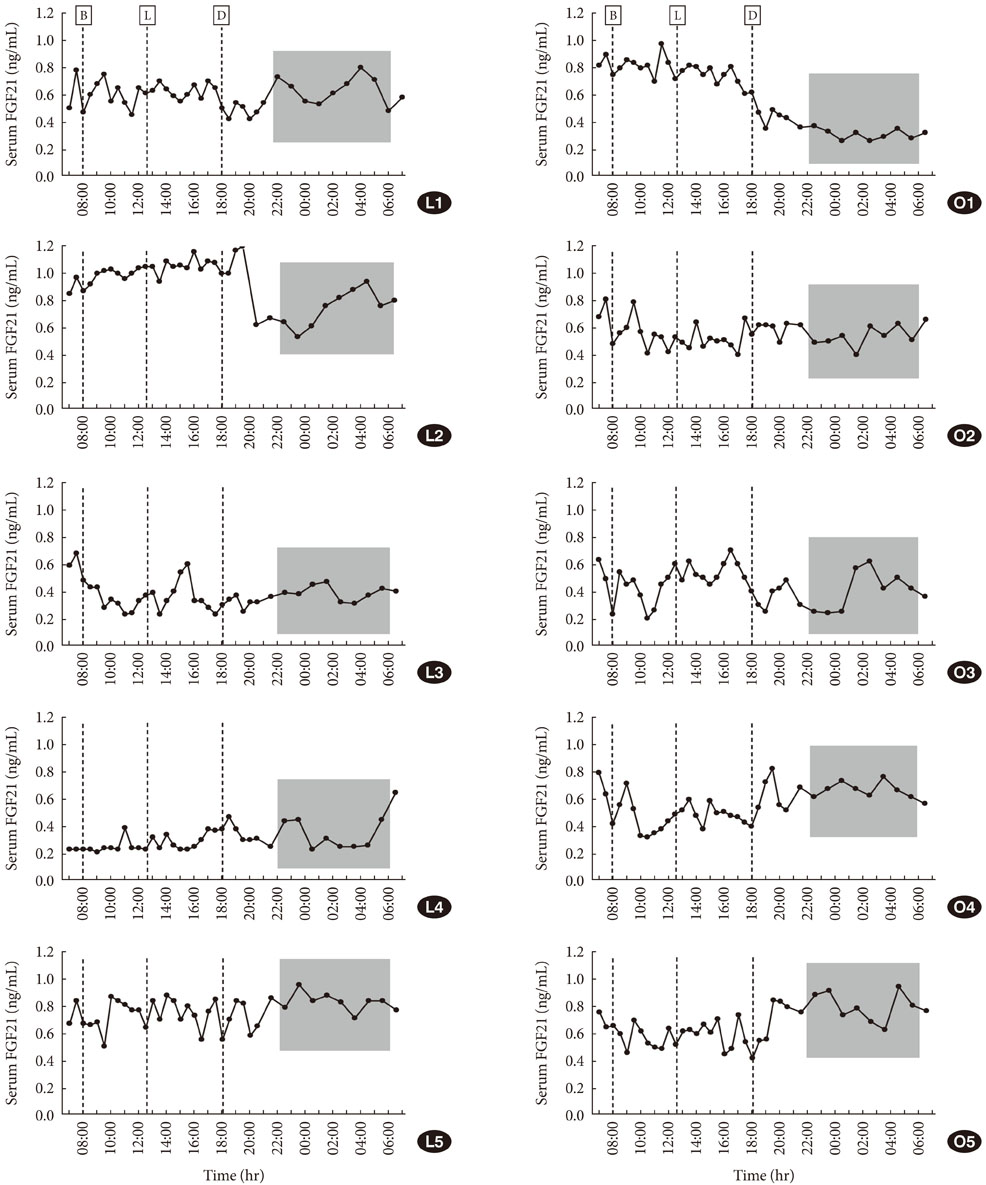
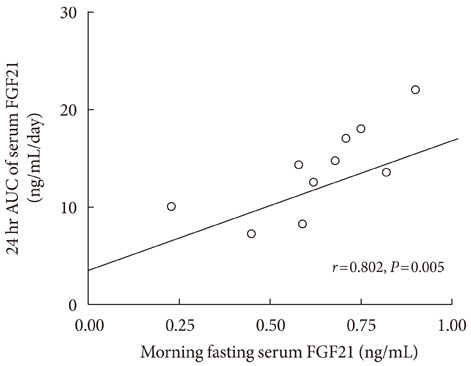
The serum FGF21 concentrations displayed various individual oscillation patterns. The oscillation frequency ranged between 6 and 12 times per day. The average duration of oscillation was 2.52 hours (range, 1.9 to 3.0 hours). The peaks and troughs of FGF21 oscillation showed no circadian rhythm. However, the oscillation frequency had a diurnal variation and was lower during the light-off period than during the light-on period (2.4 vs. 7.3 times, P<0.001). There was no difference in the total frequency or duration of oscillations between non-obese and obese subjects, but obese individuals had increased numbers of larger oscillations (amplitude > or =0.19 ng/mL).
CONCLUSION
Various oscillation patterns in serum FGF21 concentration were observed, and reduced oscillation frequencies were seen during sleep. The oscillation patterns of serum FGF21 concentration suggest that FGF21 may be secreted into systemic circulation in a pulsatile manner. Obesity appeared to affect the amplitude of oscillations of serum FGF21.
MeSH Terms
Figure
Reference
-
1. Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000. 1492:203–206.2. Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008. 74:403–412.3. Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008. 582:3805–3810.4. Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006. 281:15694–15700.5. Cuevas-Ramos D, Almeda-Valdes P, Aguilar-Salinas CA, Cuevas-Ramos G, Cuevas-Sosa AA, Gomez-Perez FJ. The role of fibroblast growth factor 21 (FGF21) on energy balance, glucose and lipid metabolism. Curr Diabetes Rev. 2009. 5:216–220.6. Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A. 2007. 104:7432–7437.7. Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, Polonsky KS, Permutt MA. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004. 114:928–936.8. Oishi K, Uchida D, Ishida N. Circadian expression of FGF21 is induced by PPARalpha activation in the mouse liver. FEBS Lett. 2008. 582:3639–3642.9. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007. 5:426–437.10. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007. 5:415–425.11. Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008. 149:6018–6027.12. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007. 148:774–781.13. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005. 115:1627–1635.14. Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2008. 8:169–174.15. Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, Tang Y, Liu H, Boden G. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008. 116:65–68.16. Durovcova V, Marek J, Hana V, Matoulek M, Zikan V, Haluzikova D, Kavalkova P, Lacinova Z, Krsek M, Haluzik M. Plasma concentrations of fibroblast growth factors 21 and 19 in patients with Cushing's syndrome. Physiol Res. 2010. 59:415–422.17. Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008. 57:1246–1253.18. Lambert AE, Hoet JJ. Diurnal pattern of plasma insulin concentration in the human. Diabetologia. 1966. 2:69–72.19. Hoffman RP. Sympathetic mechanisms of hypoglycemic counterregulation. Curr Diabetes Rev. 2007. 3:185–193.20. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001. 50:1714–1719.21. Kok SW, Meinders AE, Overeem S, Lammers GJ, Roelfsema F, Frolich M, Pijl H. Reduction of plasma leptin levels and loss of its circadian rhythmicity in hypocretin (orexin)-deficient narcoleptic humans. J Clin Endocrinol Metab. 2002. 87:805–809.22. Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010. 106:447–462.23. Estall JL, Ruas JL, Choi CS, Laznik D, Badman M, Maratos-Flier E, Shulman GI, Spiegelman BM. PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb(alpha) axis. Proc Natl Acad Sci U S A. 2009. 106:22510–22515.24. Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J Biol Chem. 2010. 285:15668–15673.25. Jeong E, Youn BS, Kim DW, Kim EH, Park JW, Namkoong C, Jeong JY, Yoon SY, Park JY, Lee KU, Kim MS. Circadian rhythm of serum vaspin in healthy male volunteers: relation to meals. J Clin Endocrinol Metab. 2010. 95:1869–1875.26. World Health Organization, International Association for the Study of Obesity, International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. 2000. Sydney: Health Communications.27. Yu H, Xia F, Lam KS, Wang Y, Bao Y, Zhang J, Gu Y, Zhou P, Lu J, Jia W, Xu A. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin Chem. 2011. 57:691–700.28. Andersen B, Beck-Nielsen H, Hojlund K. Plasma FGF21 displays a circadian rhythm during a 72-h fast in healthy female volunteers. Clin Endocrinol (Oxf). 2011. 75:514–519.29. Mai K, Andres J, Biedasek K, Weicht J, Bobbert T, Sabath M, Meinus S, Reinecke F, Mohlig M, Weickert MO, Clemenz M, Pfeiffer AF, Kintscher U, Spuler S, Spranger J. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes. 2009. 58:1532–1538.30. Dostalova I, Kavalkova P, Haluzikova D, Lacinova Z, Mraz M, Papezova H, Haluzik M. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab. 2008. 93:3627–3632.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum and Urine basic Fibroblast Growth Factor (bFGF) in Cervical Cancer Patients
- Fibroblast Growth Factor 21: A Novel Metabolic Regulator
- Association between Serum Fibroblast Growth Factor 21 Levels and Bone Mineral Density in Postmenopausal Women
- An Experimental Study of the Effect of Acidic Fibroblast Growth Factor on Nerve Regeneration
- Effects of Single Vitamin D₃ Injection (200,000 Units) on Serum Fibroblast Growth Factor 23 and Sclerostin Levels in Subjects with Vitamin D Deficiency