Diabetes Metab J.
2012 Jun;36(3):230-236. 10.4093/dmj.2012.36.3.230.
Glycemic Effects of Once-a-Day Rapid-Acting Insulin Analogue Addition on a Basal Insulin Analogue in Korean Subjects with Poorly Controlled Type 2 Diabetes Mellitus
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. bwanlee@yuhs.ac
- KMID: 2281404
- DOI: http://doi.org/10.4093/dmj.2012.36.3.230
Abstract
- BACKGROUND
The present study investigates the efficacy in glycemic control by adding once-a-day glulisine to glargine as a basal plus regimen and factors influencing glycemic control with the basal plus regimen in Korean subjects with type 2 diabetes.
METHODS
In the present retrospective study, subjects previously treated with the basal plus regimens for at least 6 months were reviewed. Changes in glycemic profiles and clinical parameters were evaluated.
RESULTS
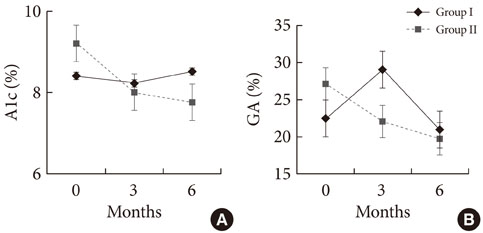
A total of 87 subjects were ultimately enrolled in this study. At baseline, mean glycated hemoglobin (A1c) and glycated albumin were 8.5% (8.0% to 9.6%) and 25.2+/-7.6%, respectively. After treatment with the basal plus regimen, patients had significant reductions of A1c at 6 months (0.8+/-0.1%, P<0.001) and their postprandial glucose levels were decreased by 48.7+/-10.3 mg/dL (P<0.001). Multiple logistic regression showed old age (odds ratio [OR], 1.25; 95% confidence interval [CI], 1.02 to 1.55), high initial A1c (OR, 22.21; 95% CI, 2.44 to 201.78), and lower amounts of glargine (OR, 0.85; 95% CI, 0.76 to 0.99), and glimepiride (OR, 0.23; 95% CI, 0.06 to 0.93) at baseline were independently associated with good responders whose A1c reduction was more than 0.5%.
CONCLUSION
The authors suggest a basal plus regimen may be effective in reducing glucose levels of subjects with old age, high initial A1c, and patients on low doses of glimepiride and glargine. Despite the use of high doses of hypoglycemic agents, elderly patients with poorly-controlled diabetes are preferred for early initiation of the basal plus regimen.
MeSH Terms
-
Aged
Diabetes Mellitus, Type 2
Glucose
Hemoglobins
Humans
Hypoglycemic Agents
Insulin
Insulin, Long-Acting
Insulin, Short-Acting
Logistic Models
Retrospective Studies
Serum Albumin
Sulfonylurea Compounds
Insulin Glargine
Glucose
Hemoglobins
Hypoglycemic Agents
Insulin
Insulin, Long-Acting
Insulin, Short-Acting
Serum Albumin
Sulfonylurea Compounds
Figure
Cited by 1 articles
-
Characteristics Predictive for a Successful Switch from Insulin Analogue Therapy to Oral Hypoglycemic Agents in Patients with Type 2 Diabetes
Gyuri Kim, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Yonsei Med J. 2016;57(6):1395-1403. doi: 10.3349/ymj.2016.57.6.1395.
Reference
-
1. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.2. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995. 28:103–117.3. ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008. 358:2560–2572.4. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. American Diabetes Association. European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009. 32:193–203.5. Groop LC, Pelkonen R, Koskimies S, Bottazzo GF, Doniach D. Secondary failure to treatment with oral antidiabetic agents in non-insulin-dependent diabetes. Diabetes Care. 1986. 9:129–133.6. Nelson SE, Palumbo PJ. Addition of insulin to oral therapy in patients with type 2 diabetes. Am J Med Sci. 2006. 331:257–263.7. Lee BW, Hur J, Yim HJ, Park JB, Woo H, Yoo HJ. Dysfunctional pancreatic beta-cells of critical stress play a more prominent role in the development of stress diabetes in critically burned Korean subjects. Metabolism. 2010. 59:1307–1315.8. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001. 50:590–593.9. Koo BK, Cho YM, Kimm K, Lee JY, Oh B, Park BL, Cheong HS, Shin HD, Ko KS, Park SG, Lee HK, Park KS. Polymorphisms of the reg1alpha gene and early onset type 2 diabetes in the Korean population. Korean Diabetes J. 2010. 34:229–236.10. Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011. 17:395–403.11. Lee YH, Lee BW, Chun SW, Cha BS, Lee HC. Predictive characteristics of patients achieving glycaemic control with insulin after sulfonylurea failure. Int J Clin Pract. 2011. 65:1076–1084.12. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008. 358:2545–2559.13. Tibaldi J, Rakel RE. Why, when and how to initiate insulin therapy in patients with type 2 diabetes. Int J Clin Pract. 2007. 61:633–644.14. Dailey G. New strategies for basal insulin treatment in type 2 diabetes mellitus. Clin Ther. 2004. 26:889–901.15. Meece J. Dispelling myths and removing barriers about insulin in type 2 diabetes. Diabetes Educ. 2006. 32:1 Suppl. 9S–18S.16. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002. 26:Suppl 3. S18–S24.17. Zambanini A, Newson RB, Maisey M, Feher MD. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract. 1999. 46:239–246.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of an Insulin Analogue in Six Hypoglycemia-Prone Hemodialysis Patients with Type 2 Diabetes
- Intraoperative and Postoperative Glycemic Management in Patients with Diabetes
- Intensive Insulin Therapy in Type 1 Diabetes
- Determining the Factors that Influence the Insulin Requirements in Type 2 Diabetic Patients
- The effect of Lantus on glycemic control in children and adolescents with type 1 diabetes mellitus