Clin Exp Vaccine Res.
2012 Jul;1(1):83-87. 10.7774/cevr.2012.1.1.83.
GFP-tagged E. coli shows bacterial distribution in mouse organs: pathogen tracking using fluorescence signal
- Affiliations
-
- 1Division of High-Risk Pathogen Research, Center for Infectious Diseases, Korea National Institute of Health, Cheongwon, Korea. khong@nih.go.kr
- 2Laboratory of Molecular Imaging and Therapy, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Nuclear Medicine, Cancer Imaging Center, Seoul National University Hospital, Seoul, Korea.
- KMID: 2278792
- DOI: http://doi.org/10.7774/cevr.2012.1.1.83
Abstract
- PURPOSE
In vaccine efficacy evaluation, visualization of pathogens in whole organism at each time point would be able to reduce the consuming animals and provide the in vivo information within consistent background with identical organism.
MATERIALS AND METHODS
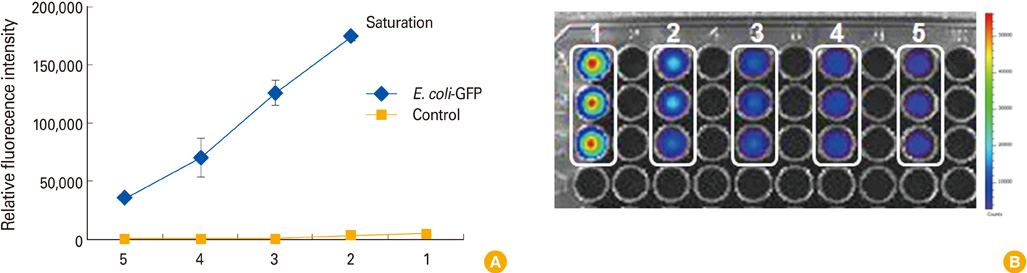
Using IVIS spectrum whole live-animal imaging system, fluorescent intensity was optimized and visualized proportionately by concentrating Escherichia coli MC1061 strain which expresses GFP (E. coli-GFP) in BALB/C mice after injection.
RESULTS
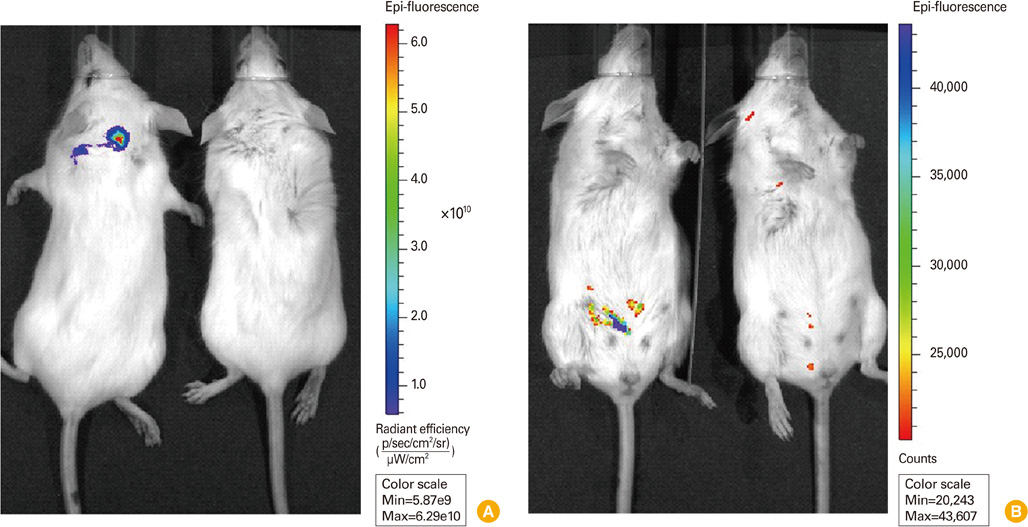
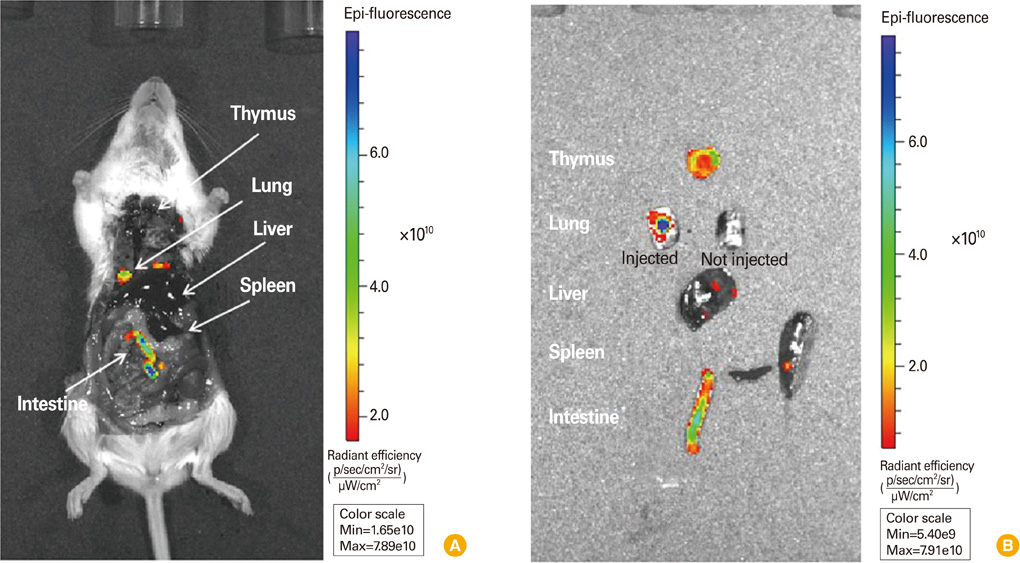
Local distribution of disseminated E. coli-GFP was traced in each organ by fluorescence. Detached organ showed more obvious fluorescent signal, and intestine showed strongest fluorescent signal.
CONCLUSION
This in vivo imaging method using GFP-tagged pathogen strain suggest quantified infected pathogens by fluorescence intensity in whole animals can provide the information about the localization and distribution after infection.
MeSH Terms
Figure
Reference
-
1. Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect Immun. 2003. 71:5940–5950.
Article2. Doyle TC, Burns SM, Contag CH. In vivo bioluminescence imaging for integrated studies of infection. Cell Microbiol. 2004. 6:303–317.
Article3. Shen H, Harris G, Chen W, Sjostedt A, Ryden P, Conlan W. Molecular immune responses to aerosol challenge with Francisella tularensis in mice inoculated with live vaccine candidates of varying efficacy. PLoS One. 2010. 5:e13349.
Article4. Nham T, Filali S, Danne C, Derbise A, Carniel E. Imaging of bubonic plague dynamics by in vivo tracking of bioluminescent Yersinia pestis. PLoS One. 2012. 7:e34714.
Article5. Sanz P, Teel LD, Alem F, Carvalho HM, Darnell SC, O'Brien AD. Detection of Bacillus anthracis spore germination in vivo by bioluminescence imaging. Infect Immun. 2008. 76:1036–1047.
Article6. Green M, Choules G, Rogers D, Titball RW. Efficacy of the live attenuated Francisella tularensis vaccine (LVS) in a murine model of disease. Vaccine. 2005. 23:2680–2686.
Article7. Bina XR, Miller MA, Bina JE. Construction of a bioluminescence reporter plasmid for Francisella tularensis. Plasmid. 2010. 64:156–161.
Article8. Miller MA, Stabenow JM, Parvathareddy J, et al. Visualization of murine intranasal dosing efficiency using luminescent Francisella tularensis: effect of instillation volume and form of anesthesia. PLoS One. 2012. 7:e31359.
Article9. Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002. 4:235–260.
Article10. Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005. 2:905–909.
Article11. Youn H, Hong KJ. In vivo noninvasive small animal molecular imaging. Osong Public Health Res Perspect. 2012. 3:48–59.12. Kawasaki ES, Player A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine. 2005. 1:101–109.
Article13. Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006. 124:849–863.
Article14. Pechous RD, McCarthy TR, Zahrt TC. Working toward the future: insights into Francisella tularensis pathogenesis and vaccine development. Microbiol Mol Biol Rev. 2009. 73:684–711.
Article15. Hagenaars N, Mania M, de Jong P, et al. Role of trimethylated chitosan (TMC) in nasal residence time, local distribution and toxicity of an intranasal influenza vaccine. J Control Release. 2010. 144:17–24.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unfolded Histidine-Tagged Protein is Immobilized to Nitrilotriacetic Acid-Nickel Beads, But Not the Nickel-Coated Glass Slide
- Development of dual reporter imaging system for Francisella tularensis to monitor the spatio-temporal pathogenesis and vaccine efficacy
- Expression of Green Fluorescent Protein in Both Spodoptera frugiperda Cells and Bombyx mori Larvae by Ac-Bm Hybrid Virus
- Tracking the Fate of Muscle-derived Stem Cells: an Insight into the Distribution and Mode of Action
- In vitro MRI and Characterization of Rat Mesenchymal Stem Cells Transduced with Ferritin as MR Reporter Gene