Chonnam Med J.
2012 Apr;48(1):21-26. 10.4068/cmj.2012.48.1.21.
Body Mass Index and Nonresponse to Antiviral Treatment in Korean Patients with Genotype 2 and 3 Chronic Hepatitis C
- Affiliations
-
- 1Department of Internal Medicine, Chonnam University Medical School, Gwangju, Korea. choisk@chonnam.ac.kr
- KMID: 2274877
- DOI: http://doi.org/10.4068/cmj.2012.48.1.21
Abstract
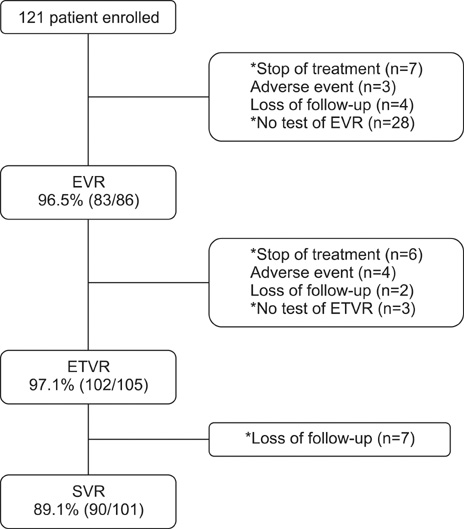
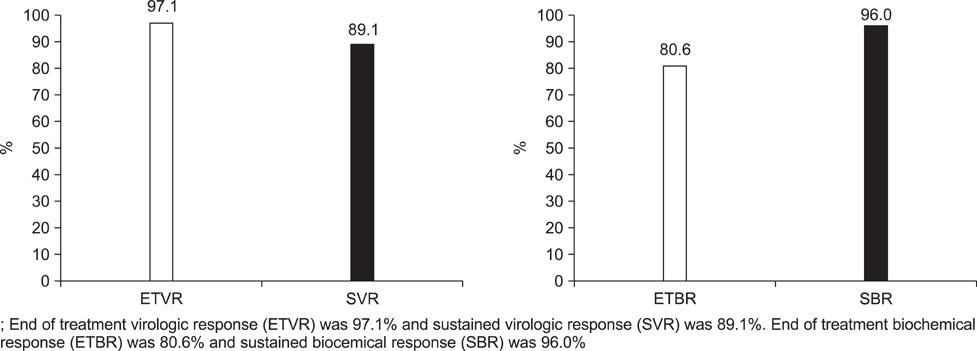
- Pegylated-interferon plus ribavirin is the standard treatment for chronic hepatitis C. Sustained virological response (SVR) rates of up to 80% are reported in genotype 2 and 3 chronic hepatitis C cases. Obesity, a modifiable risk factor, may have a deleterious effect on antiviral treatment. We performed this study to examine the efficacy and safety of pegylated-interferon and ribavirin therapy in Korean patients with genotype 2 and 3 chronic hepatitis C and to investigate the risk factors for nonresponse to antiviral treatment. A total of 121 patients were treated with peginterferon alpha-2a 180 mcg/week plus ribavirin 800 mg/day for 24 weeks. The end-of-treatment virologic response (ETVR), the SVR, the end-of-treatment biochemical response (ETBR), the sustained biochemical response (SBR), and the adverse events were analyzed. The ETVR and SVR were 94.1% and 89.1%, respectively. The ETBR was 80.2% and the SBR was 96%. Multivariate analysis showed that a body mass index of 25 and over was the only independent factor that affected the SVR (odds ratio=10.5, 95% confidence interval: 2.006-54.948, p=0.005). Twenty patients (16.5%) dropped out at the end of treatment, and 7 (5.8%) patients discontinued treatment because of treatment-related adverse events. Our study showed that combination therapy with pegylated-interferon and ribavirin as an initial treatment for genotype 2 and 3 chronic hepatitis C is very effective and safe, and that body mass index is an independent risk factor for nonresponse to antiviral treatment in patients with genotype 2 and 3 chronic hepatitis C.
MeSH Terms
Figure
Reference
-
1. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000. 31:1014–1018.
Article2. Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001. 345:41–52.
Article3. WHO. Hepatitis C: global prevalence (update).4. Kim YS, Pai CH, Chi HS, Kim DW, Min YI, Ahn YO. Prevalence of hepatitis C virus antibody among Korean adults. J Korean Med Sci. 1992. 7:333–336.
Article5. Lee YS, Chung YH, Min YI, Moon DH, Na DS, Suh DJ. Hepatitis C virus genotyping of 100 consecutive anti-HCV positive cases with PCR using type=specific primers. Korean J Hepatol. 1998. 4:235–243.6. Sievert W. Management issues in chronic viral hepatitis: hepatitis C. J Gastroenterol Hepatol. 2002. 17:415–422.
Article7. Strader DB, Wright T, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004. 39:1147–1171.
Article8. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001. 358:958–965.
Article9. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002. 347:975–982.
Article10. NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002. 19:1–46.11. Charlton MR, Pockros PJ, Harrison SA. Impact of obesity on treatment of chronic hepatitis C. Hepatology. 2006. 43:1177–1186.
Article12. Lee H, Choi MS, Paik SW, Kim JH, Kim DY, Lee JH, et al. Peginterferon alfa-2a plus ribavirin for initial treatment of chronic hepatitis C in Korea. Korean J Hepatol. 2006. 12:31–40.13. Hwang SY, Lee HJ, Park KT, Kim KY, Lee SM, Park CW, et al. Effectiveness and complications of combination therapy with interferon alpha and ribavirin in patients with chronic hepatitis C. Korean J Gastroenterol. 2007. 49:166–172.14. Lee HJ, Eun JR, Choi JW, Kim KO, Moon HJ. Comparison of therapeutic results between combination therapy of peginterferon alpha-2a plus ribavirin and interferon alpha-2b plus ribavirin according to treatment duration in patients with chronic hepatitis C. Korean J Hepatol. 2008. 14:46–57.
Article15. Missiha S, Heathcote J, Arenovich T, Khan K. Canadian Pegasys Expanded Access Group. Impact of asian race on response to combination therapy with peginterferon alfa-2a and ribavirin in chronic hepatitis C. Am J Gastroenterol. 2007. 102:2181–2188.
Article16. Pattullo V, Heathcote EJ, Wong DK. Superior response to pegylated interferon and ribavirin in Asians with chronic hepatitis C. Hepatol Int. 2010. 4:723–731.
Article17. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009. 461:399–401.
Article18. Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009. 41:1105–1109.
Article19. Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A, et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999. 29:1215–1219.
Article20. Clouston AD, Jonsson JR, Purdie DM, Macdonald GA, Pandeya N, Shorthouse C, et al. Steatosis and chronic hepatitis C: analysis of fibrosis and stellate cell activation. J Hepatol. 2001. 34:314–320.
Article21. Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000. 343:1666–1672.
Article22. Wellens RI, Roche AF, Khamis HJ, Jackson AS, Pollock ML, Siervogel RM. Relationships between the Body Mass Index and body composition. Obes Res. 1996. 4:35–44.
Article23. Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003. 38:639–644.
Article24. Everhart JE, Lok AS, Kim HY, Morgan TR, Lindsay KL, Chung RT, et al. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology. 2009. 137:549–557.
Article25. Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001. 33:1358–1364.
Article26. Porter CJ, Charman SA. Lymphatic transport of proteins after subcutaneous administration. J Pharm Sci. 2000. 89:297–310.
Article27. Banerjee D, Williams EV, Ilott J, Monypenny IJ, Webster DJ. Obesity predisposes to increased drainage following axillary node clearance: a prospective audit. Ann R Coll Surg Engl. 2001. 83:268–271.28. Yu ML, Dai CY, Chen SC, Chiu CC, Lee LP, Lin ZY, et al. Human leukocyte antigen class I and II alleles and response to interferon-alpha treatment, in Taiwanese patients with chronic hepatitis C virus infection. J Infect Dis. 2003. 188:62–65.
Article29. Persico M, Capasso M, Russo R, Persico E, Crocè L, Tiribelli C, et al. Elevated expression and polymorphisms of SOCS3 influence patient response to antiviral therapy in chronic hepatitis C. Gut. 2008. 57:507–515.
Article30. Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003. 124:1711–1719.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New antiviral agents for treatment of chronic hepatitis B
- Antiviral Therapy in Patients after Treatment for Hepatitis C-Related Hepatocellular Carcinoma
- Treatment of chronic hepatitis C
- 2017 Korean Association for the Study of the Liver (KASL) Clinical Practice Guidelines of Chronic Hepatitis C: What's New?
- Factors Associated with Improved Glycemic Control by Direct-Acting Antiviral Agent Treatment in Egyptian Type 2 Diabetes Mellitus Patients with Chronic Hepatitis C Genotype 4