Ann Dermatol.
2013 Aug;25(3):340-347. 10.5021/ad.2013.25.3.340.
Differentiation of Benign Pigmented Skin Lesions with the Aid of Computer Image Analysis: A Novel Approach
- Affiliations
-
- 1Department of Dermatology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. swyoun@snu.ac.kr
- KMID: 2265875
- DOI: http://doi.org/10.5021/ad.2013.25.3.340
Abstract
- BACKGROUND
The differential diagnosis of common pigmented skin lesions is important in cosmetic dermatology. The computer aided image analysis would be a potent ancillary diagnostic tool when patients are hesitant to undergo a skin biopsy.
OBJECTIVE
We investigated the numerical parameters discriminating each pigmented skin lesion from another with statistical significance.
METHODS
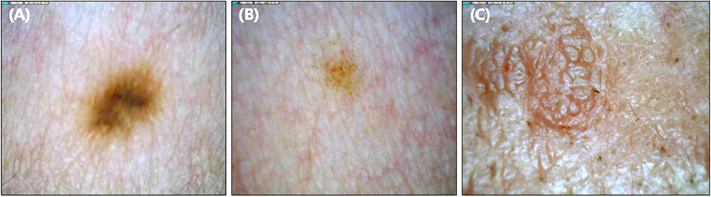
For each of the five magnified digital images containing clinically diagnosed nevus, lentigo and seborrheic keratosis, a total of 23 parameters describing the morphological, color, texture and topological features were calculated with the aid of a self-developed image analysis software. A novel concept of concentricity was proposed, which represents how closely the color segmentation resembles a concentric circle.
RESULTS
Morphologically, seborrheic keratosis was bigger and spikier than nevus and lentigo. The color histogram revealed that nevus was the darkest and had the widest variation in tone. In the aspect of texture, the surface of the nevus showed the highest contrast and correlation. Finally, the color segmented pattern of the nevus and lentigo was far more concentric than that of seborrheic keratosis.
CONCLUSION
We found that the subtle distinctions between nevus, lentigo and seborrheic keratosis, which are likely to be unrecognized by ocular inspection, are well emphasized and detected with the aid of software.
Keyword
MeSH Terms
Figure
Reference
-
1. Bogdan Allemann I, Goldberg DJ. Benign pigmented lesions. Curr Probl Dermatol. 2011; 42:81–96.
Article2. van Ginneken B, ter Haar Romeny BM, Viergever MA. Computer-aided diagnosis in chest radiography: a survey. IEEE Trans Med Imaging. 2001; 20:1228–1241.
Article3. van Ginneken B, Schaefer-Prokop CM, Prokop M. Computer-aided diagnosis: how to move from the laboratory to the clinic. Radiology. 2011; 261:719–732.
Article4. Vestergaard ME, Menzies SW. Automated diagnostic instruments for cutaneous melanoma. Semin Cutan Med Surg. 2008; 27:32–36.
Article5. Armstrong BK, Kricker A. Cutaneous melanoma. Cancer Surv. 1994; 19-20:219–240.6. Scherg M, Brinkmann RD. Least-square-fit technique applied to the frequency following potential: a method to determine components, latencies and amplitudes. Scand Audiol Suppl. 1979; (9):197–203.7. Pribić R. Principal component analysis of Fourier transform infrared and/or circular dichroism spectra of proteins applied in a calibration of protein secondary structure. Anal Biochem. 1994; 223:26–34.
Article8. Stoffel JC. Graphical and binary image processing and applications. Dedham, MA: Artech House;1982. p. 580.9. Lee T, Ng V, Gallagher R, Coldman A, McLean D. Dull-Razor: a software approach to hair removal from images. Comput Biol Med. 1997; 27:533–543.
Article10. Kiani K, Sharafat AR. E-shaver: an improved DullRazor(®) for digitally removing dark and light-colored hairs in dermoscopic images. Comput Biol Med. 2011; 41:139–145.
Article11. Abbas Q, Celebi ME, Fondón García I, Rashid M. Lesion border detection in dermoscopy images using dynamic programming. Skin Res Technol. 2011; 17:91–100.
Article12. LeAnder R, Chindam P, Das M, Umbaugh SE. Differentiation of melanoma from benign mimics using the relative-color method. Skin Res Technol. 2010; 16:297–304.
Article13. Pantic I, Pantic S, Basta-Jovanovic G. Gray level co-occurrence matrix texture analysis of germinal center light zone lymphocyte nuclei: physiology viewpoint with focus on apoptosis. Microsc Microanal. 2012; 18:470–475.
Article14. Yu S, Tranchevent LC, Liu X, Glänzel W, Suykens JA, De Moor B, et al. Optimized data fusion for kernel k-means clustering. IEEE Trans Pattern Anal Mach Intell. 2012; 34:1031–1039.
Article15. Happle R. What is a nevus? A proposed definition of a common medical term. Dermatology. 1995; 191:1–5.16. Pinkus H. Keratosis senilis; a biologic concept of its pathogenesis and diagnosis based on the study of normal epidermis and 1730 seborrheic and senile keratoses. Am J Clin Pathol. 1958; 29:193–207.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differences in the Known Cellular Composition of Benign Pigmented Skin Lesions Reflected in Computer-Aided Image Analysis
- Diagnostic Trial of Epiluminescence Microscopy in Two Cases of Pigmented Basal Cell Carcinomas (PBCCs)
- A Case of Pigmented Neurofibroma
- Two Cases of Multicentric Pigmented Bowen's Disease
- A comparative study between simple enumeratio and computerized image analysis of AgNOR in melanocytic skin lesions