Korean J Hematol.
2007 Dec;42(4):433-438. 10.5045/kjh.2007.42.4.433.
Hypercalcemia and Extensive Osteolytic Lesion with Increased Plasma Prostaglandin E2 Level in a Child with Acute Lymphoblastic Leukemia
- Affiliations
-
- 1Department of Pediatrics, Hanyang University College of Medicine, Seoul, Korea. cord@hanyang.ac.kr
- KMID: 2252233
- DOI: http://doi.org/10.5045/kjh.2007.42.4.433
Abstract
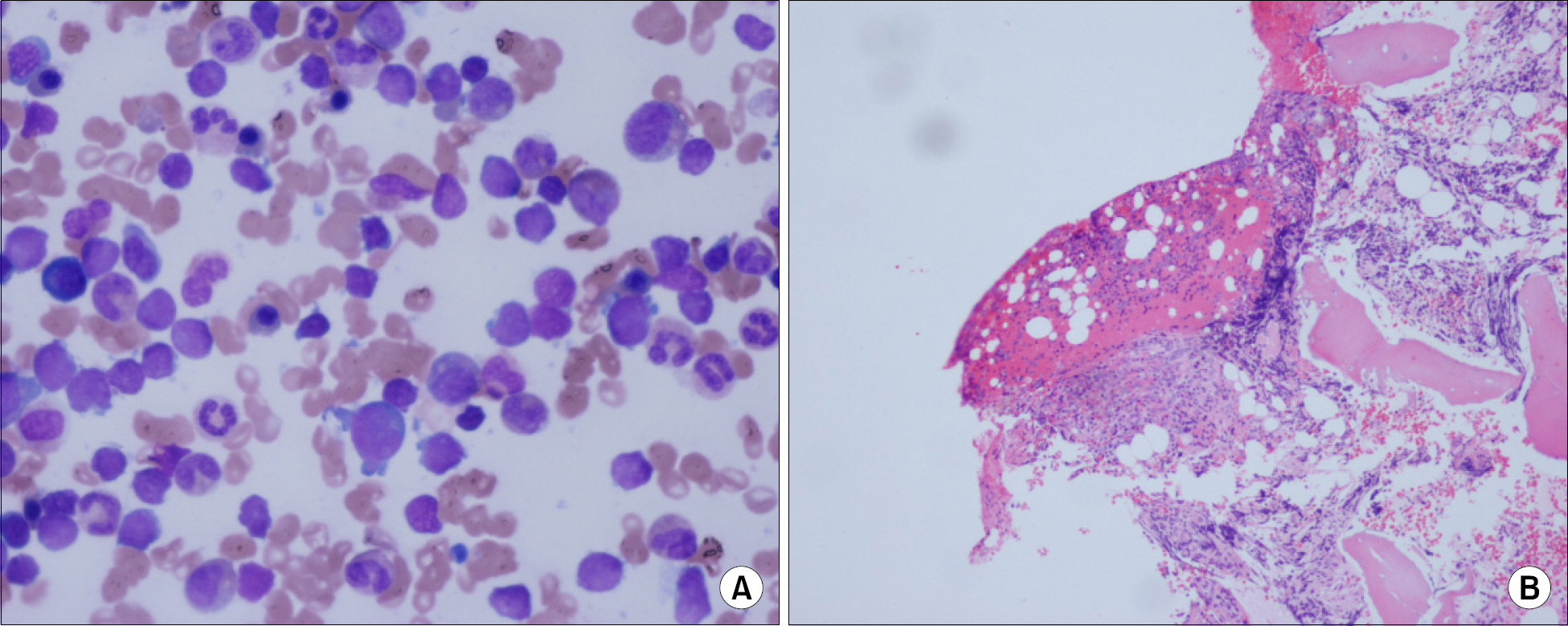
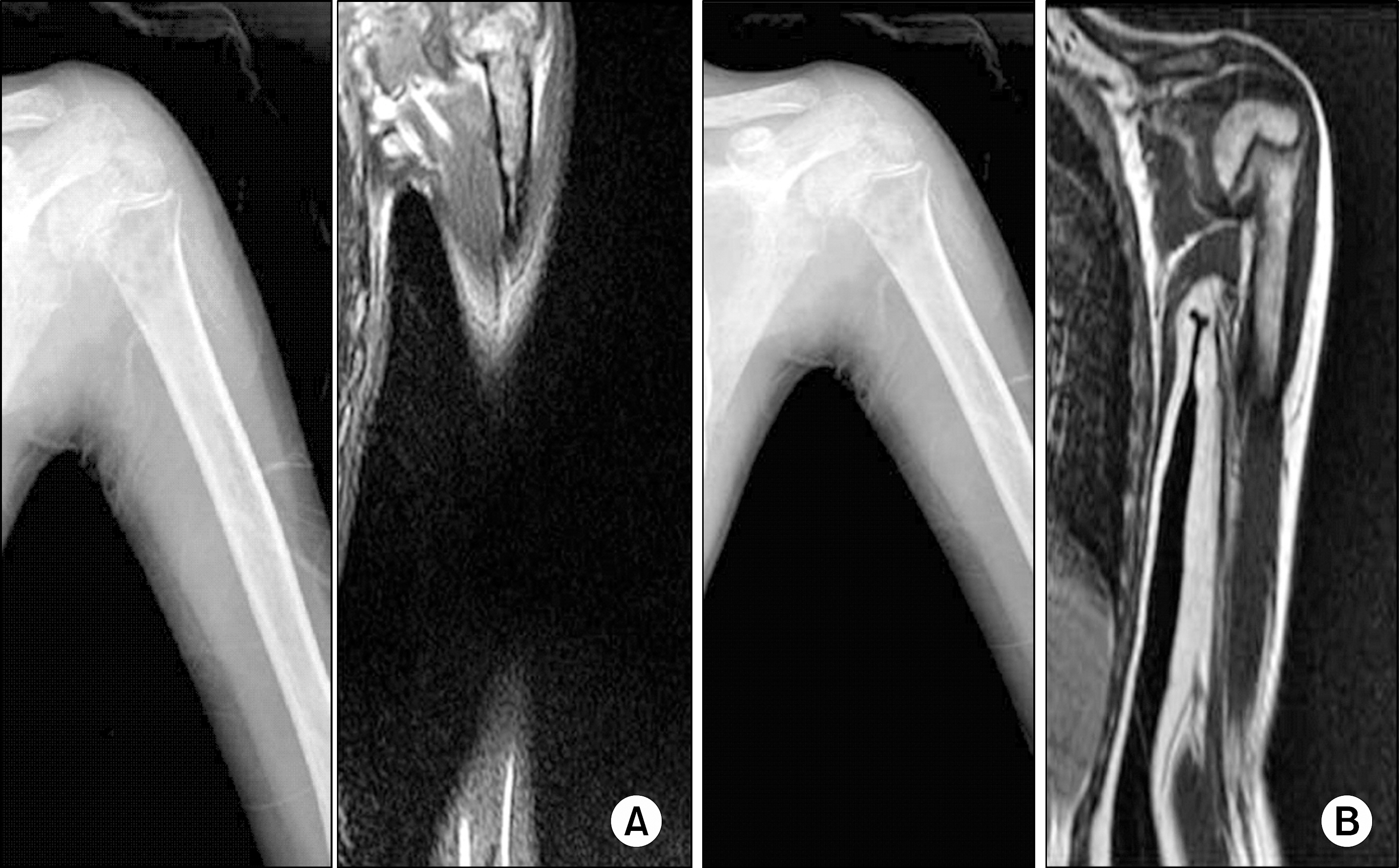
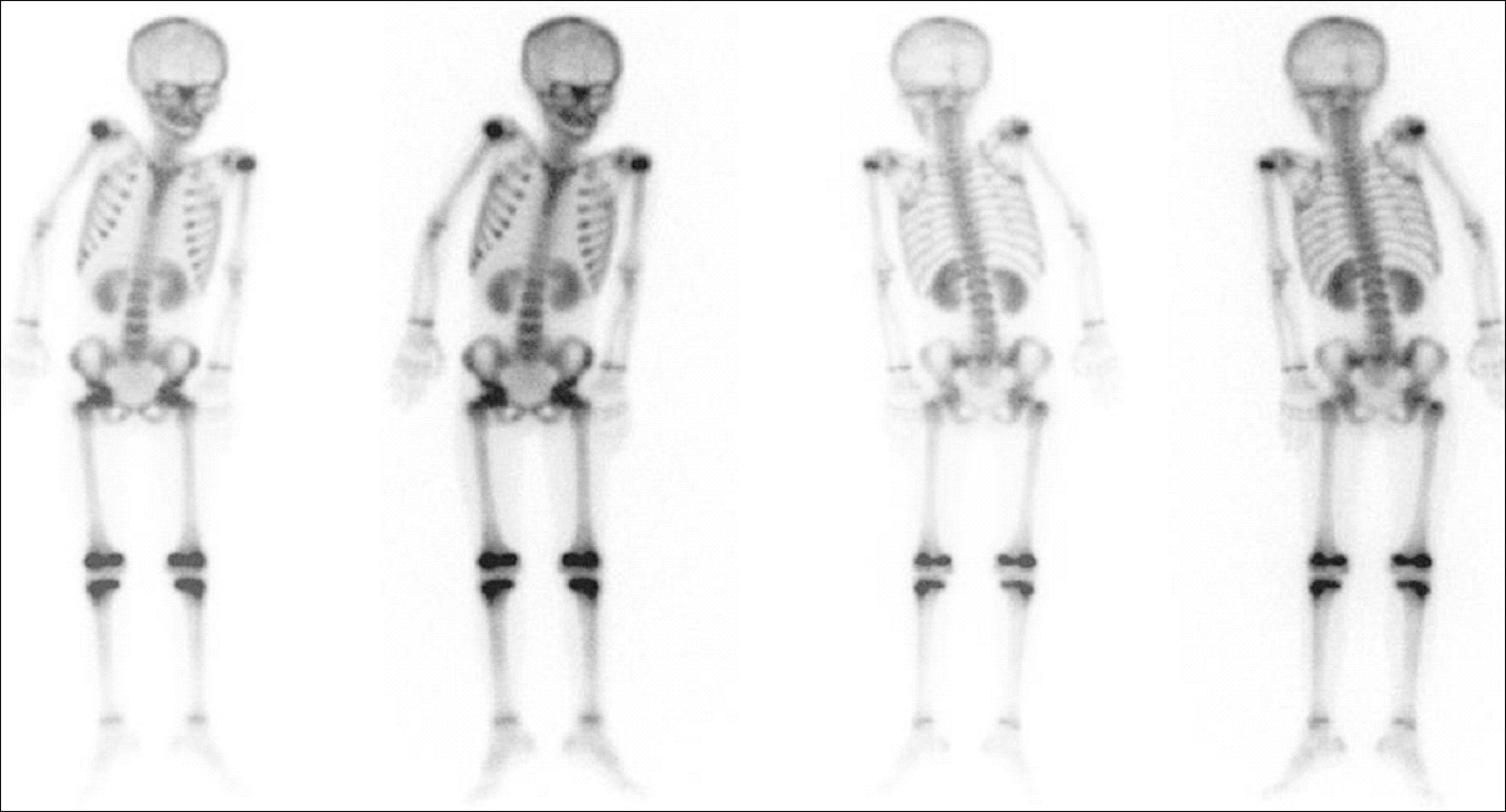
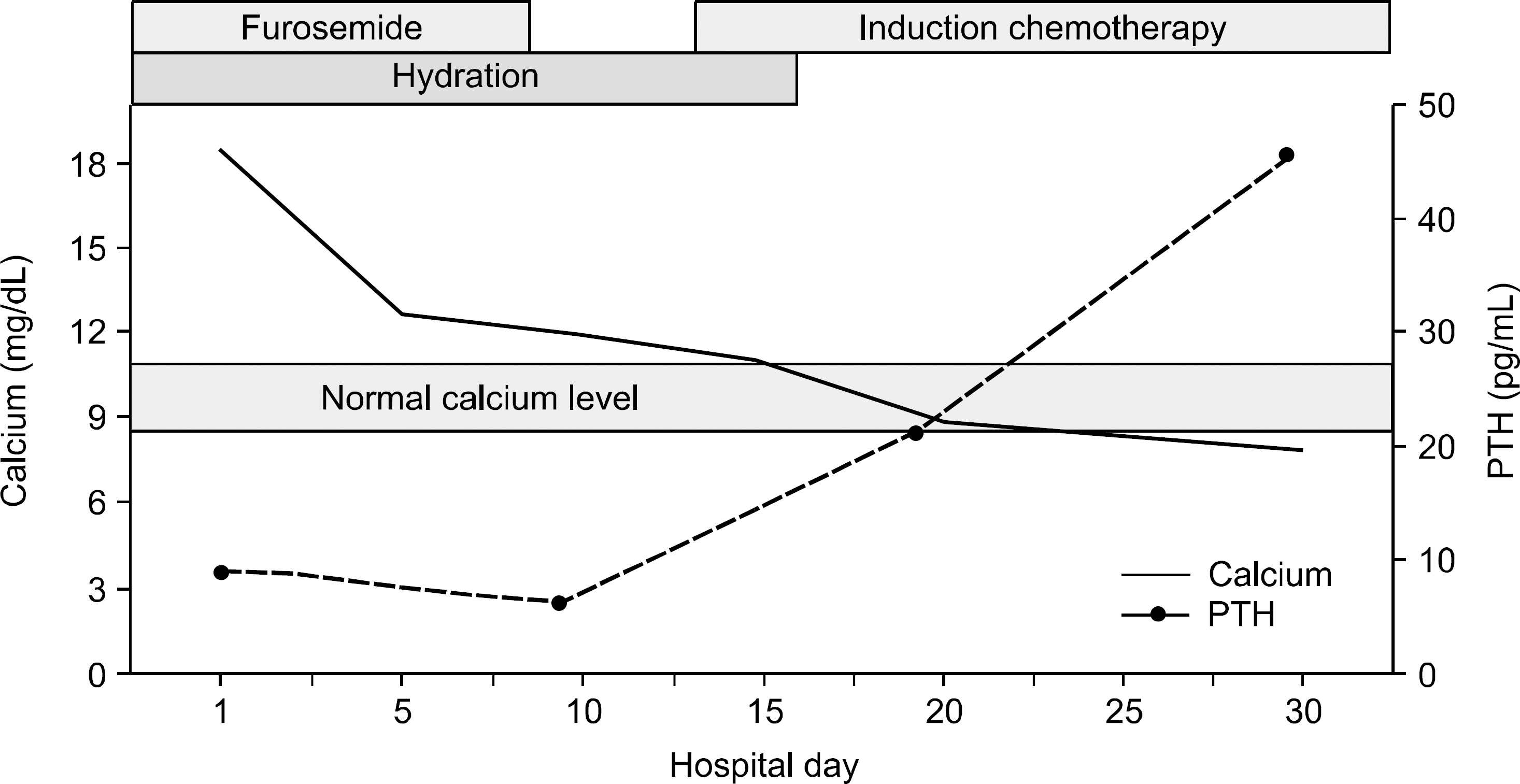
- In this report, we present a rare case of childhood ALL with hypercalcemia and extensive osteolytic lesions. The case was a 7-year-old girl presenting with vomiting and aggravating bone pain. Radiologic examinations showed severe osteolytic lesions of the skull and extremities. Laboratory findings revealed low hemoglobin, normal WBC count with absent circulating blasts, and an increased serum calcium level. Serum intact PTH and 1,25-(OH)2 vitamin D3 levels were below the normal ranges and parathyroid hormone-related peptide (PTHrP) was not detected, whereas serum levels of prostaglandin E2 were elevated. The hypercalcemia resolved with specific antileukemic chemotherapy along with supportive care. The elevated plasma prostaglandin E2 levels decreased slightly after complete remission with induction chemotherapy. These findings suggest that increased plasma prostaglandin E2 levels may be one of the pathogenetic mechanisms responsible for the occurrence of hypercalcemia in this patient.
MeSH Terms
Figure
Cited by 1 articles
-
A successful treatment of hypercalcemia with zoledronic acid in a 15-year-old boy with acute lymphoblastic leukemia
Hye-Jin Park, Eun-Jin Choi, Jin-Kyung Kim
Ann Pediatr Endocrinol Metab. 2016;21(2):99-104. doi: 10.6065/apem.2016.21.2.99.
Reference
-
1). McKay C., Furman WL. Hypercalcemia complicating childhood malignancies. Cancer. 1993. 72:256–60.
Article2). Hibi S., Funaki H., Ochiai-Kanai R, et al. Hypercalcemia in children presenting with acute lymphoblastic leukemia. Int J Hematol. 1997. 66:353–7.3). Soni PN. Hypercalcemia and multiple osteolytic lesions in childhood acute lymphoblastic leukaemia. Postgrad Med J. 1993. 69:483–5.4). Firkin F., Schneider H., Grill V. Parathyroid hormone-related protein in hypercalcemia associated with hematological malignancy. Leuk Lymphoma. 1998. 29:499–506.
Article5). Esbrit P. Hypercalcemia of malignancy - New insights into an old syndrome. Clin Lab. 2001. 47:67–71.6). Wysolmerski JJ., Broadus AE. Hypercalcemia of malignancy: the central role of parathyroid hormone-related protein. Ann Rev Med. 1994. 45:189–200.
Article7). Jacobson JO., Bringhurst FR., Harris NL., Weitzman SA., Aisenberg AC. Humoral hypercalcemia in Hodgkin's disease. Clinical and laboratory evaluation. Cancer. 1989. 63:917–23.
Article8). Todo S., Imashuku S., Inoda H, et al. Hypercalcemia in a case of childhood acute lymphoblastic leukemia. Jpn J Clin Oncol. 1987. 17:357–62.9). Antunovic P., Marisavljevic D., Kraguljac N., Jelusic V. Severe hypercalcemia and extensive osteolytic lesions in an adult patient with T cell acute lymphoblastic leukaemia. Med Oncol. 1998. 15:58–60.10). Tamura T., Udagawa N., Takahashi N, et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci USA. 1993. 90:11924–8.
Article11). Sanduja SK., Trial J., Hall ER. Prostaglandin production by human promyelocytic leukemia (HL-60) cells. Biomed Biochim Acta. 1988. 47:383–93.12). Wark JD., Taft JL., Michaelangeli VP., Veroni MC., Larkins RG. Biphasic action of prostaglandin E2 on conversion of 25-hydroxyvitamin D3 to 1, 25-dihy-droxyvitamin D3 in chick renal tubules. Prostaglandins. 1984. 27:453–63.13). Franklin RB., Tashjian AH Jr. Intravenous infusion of prostaglandin E2 raises plasma calcium concentration in the rat. Endocrinology. 1975. 97:240–3.14). Shimonodan H., Nagayama J., Nagatoshi Y, et al. Acute lymphocytic leukemia inadolescence with multiple osteolytic lesions and hypercalcemia mediated by lymphoblast-producing parathyroid hormone-related peptide: a case report and review of the literature. Pediatr Blood Cancer. 2005. 45:333–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A successful treatment of hypercalcemia with zoledronic acid in a 15-year-old boy with acute lymphoblastic leukemia
- Acute Lymphoblastic Leukemia associated with Hypercalcemia and Geralized Visceal Calcification
- Hypercalcemia, multiple osteolytic lesions, and acute renal failure: a rare presentation of B-cell acute lymphoblastic leukemia
- Children with Osteolytic Lesions – Enigmatic Presentation of a Life-Threatening Condition: Case Report and Literature Review
- A Case of Severe Hypercalcemia Causing Acute Kidney Injury: An Unusual Presentation of Acute Lymphoblastic Leukemia