J Breast Cancer.
2012 Jun;15(2):189-196. 10.4048/jbc.2012.15.2.189.
The Predictive Value of Serum HER2/neu for Response to Anthracycline-Based and Trastuzumab-Based Neoadjuvant Chemotherapy
- Affiliations
-
- 1Department of Surgery, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 2Division of Breast-Endocrine Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. brdrson@korea.com
- KMID: 2242189
- DOI: http://doi.org/10.4048/jbc.2012.15.2.189
Abstract
- PURPOSE
Little information exists about the possible influence of serum HER2/neu on response to chemotherapy. We propose that the assessment of serum HER2/neu in a pretreatment serum sample may be useful in predicting response to neoadjuvant chemotherapy.
METHODS
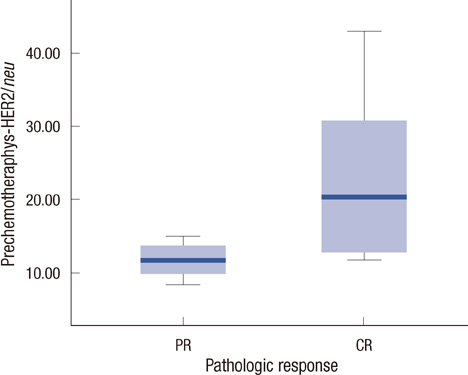
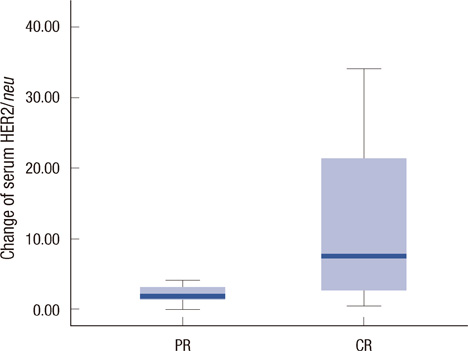
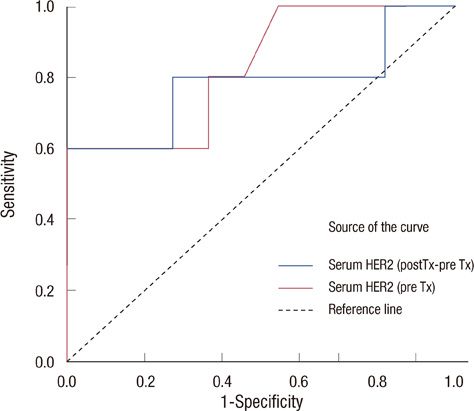
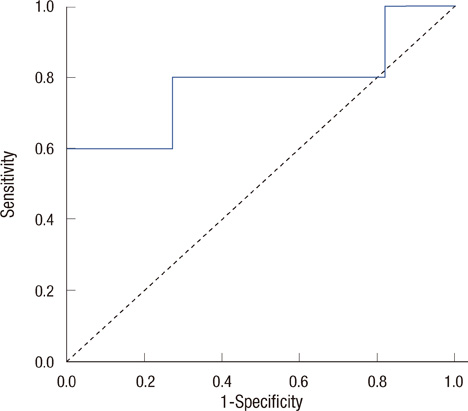
All breast cancer patients were tested by immunohistochemical stain and fluorescent in situ hybridization for HER2/neu before treatment. Serum HER2/neu was twice measured by chemiluminescence immunoassay (ADVIA Centaur System) before neoadjuvant chemotherapy and before operation. The cut-off value was 10.2 mg/mL, according to the previous study. Pathologic complete response (pCR) was considered as no residual tumor or remnant ductal carcinoma in situ; partial response (PR) was a less than 50% decrease in maximal diameter in pathologic tumor size. The measurements for the changes of serum HER2/neu were defined as pretreatment HER2/neu-preoperation HER2/neu. We compared the change of serum HER2/neu between that from before chemotherapy and that after chemotherapy, the pathologic complete response and partial response, and the trastuzumab group and anthracycline group.
RESULTS
Serum HER2/neu was decreased after neoadjuvant chemotherapy. The mean of serum HER2/neu in prechemotherapy was 15.4+/-9.0 ng/mL, and that of postchemotherapy was 10.5+/-2.0 ng/mL (p=0.04). Pathologic response was correlated with the change of serum HER2/neu (PR, 11.7+/-2.2 ng/mL vs. pCR, 23.7+/-13.1 ng/mL; p=0.01). In the trastuzumab group, pCR was marginally correlated with the change of serum HER2/neu (PR, 0.8+/-0.84 ng/mL vs. pCR, 21.1+/-13.2 ng/mL; p=0.08).
CONCLUSION
Serum HER2/neu levels during treatment were associated with pathologic response in patients receiving neoadjuvant chemotherapy, particularly, in a trastuzumab-based regimen. The change of serum HER2/neu levels may serve in monitoring neoadjuvant therapy in HER2/neu-overexpressed breast cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985. 230:1132–1139.
Article2. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987. 235:177–182.
Article3. Agrup M, Stål O, Olsen K, Wingren S. C-erbB-2 overexpression and survival in early onset breast cancer. Breast Cancer Res Treat. 2000. 63:23–29.
Article4. Zabrecky JR, Lam T, McKenzie SJ, Carney W. The extracellular domain of p185/neu is released from the surface of human breast carcinoma cells, SK-BR-3. J Biol Chem. 1991. 266:1716–1720.
Article5. Narita T, Funahashi H, Satoh Y, Takagi H. C-erbB-2 protein in the sera of breast cancer patients. Breast Cancer Res Treat. 1992. 24:97–102.
Article6. Molina R, Jo J, Filella X, Zanon G, Pahisa J, Muñoz M, et al. c-erbB-2 oncoprotein, CEA, and CA 15.3 in patients with breast cancer: prognostic value. Breast Cancer Res Treat. 1998. 51:109–119.
Article7. Fehm T, Maimonis P, Weitz S, Teramoto Y, Katalinic A, Jäger W. Influence of circulating c-erbB-2 serum protein on response to adjuvant chemotherapy in node-positive breast cancer patients. Breast Cancer Res Treat. 1997. 43:87–95.
Article8. Leitzel K, Teramoto Y, Konrad K, Chinchilli VM, Volas G, Grossberg H, et al. Elevated serum c-erbB-2 antigen levels and decreased response to hormone therapy of breast cancer. J Clin Oncol. 1995. 13:1129–1135.
Article9. Yamauchi H, O'Neill A, Gelman R, Carney W, Tenney DY, Hösch S, et al. Prediction of response to antiestrogen therapy in advanced breast cancer patients by pretreatment circulating levels of extracellular domain of the HER-2/c-neu protein. J Clin Oncol. 1997. 15:2518–2525.
Article10. Paik S, Bryant J, Park C, Fisher B, Tan-Chiu E, Hyams D, et al. erbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptor-negative breast cancer. J Natl Cancer Inst. 1998. 90:1361–1370.
Article11. Kostler WJ, Schwab B, Singer CF, Neumann R, Rücklinger E, Brodowicz T, et al. Monitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res. 2004. 10:1618–1624.
Article12. Mazouni C, Hall A, Broglio K, Fritsche H, Andre F, Esteva FJ, et al. Kinetics of serum HER-2/neu changes in patients with HER-2-positive primary breast cancer after initiation of primary chemotherapy. Cancer. 2007. 109:496–501.
Article13. Kong SY, Kang JH, Kwon Y, Kang HS, Chung KW, Kang SH, et al. Serum HER-2 concentration in patients with primary breast cancer. J Clin Pathol. 2006. 59:373–376.
Article14. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.15. Cook GB, Neaman IE, Goldblatt JL, Cambetas DR, Hussain M, Lüftner D, et al. Clinical utility of serum HER-2/neu testing on the Bayer Immuno 1 automated system in breast cancer. Anticancer Res. 2001. 21(2B):1465–1470.16. Pallud C, Guinebretiere JM, Guepratte S, Hacene K, Neumann R, Carney W, et al. Tissue expression and serum levels of the oncoprotein HER-2/neu in 157 primary breast tumours. Anticancer Res. 2005. 25(2B):1433–1440.17. Saghatchian M, Guepratte S, Hacene K, Neumann R, Floiras JL, Pichon MF. Serum HER-2 extracellular domain: relationship with clinicobiological presentation and prognostic value before and after primary treatment in 701 breast cancer patients. Int J Biol Markers. 2004. 19:14–22.
Article18. Colomer R, Montero S, Lluch A, Ojeda B, Barnadas A, Casado A, et al. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 2000. 6:2356–2362.19. Müller V, Witzel I, Lück HJ, Köhler G, von Minckwitz G, Möbus V, et al. Prognostic and predictive impact of the HER-2/ neu extracellular domain (ECD) in the serum of patients treated with chemotherapy for metastatic breast cancer. Breast Cancer Res Treat. 2004. 86:9–18.
Article20. Bast RC Jr, Ravdin P, Hayes DF, Bates S, Fritsche H Jr, Jessup JM, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001. 19:1865–1878.
Article21. Lennon S, Barton C, Banken L, Gianni L, Marty M, Baselga J, et al. Utility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in metastatic breast cancer. J Clin Oncol. 2009. 27:1685–1693.
Article22. Ali SM, Leitzel K, Lipton A, Carney WP, Kostler WJ. Value of serum human epidermal growth factor receptor 2 (HER2)/neu testing for early prediction of response to HER2/neu-directed therapies is still an open one and deserves further study in large prospective trials. J Clin Oncol. 2009. 27:e273.23. Leary AF, Hanna WM, van de Vijver MJ, Penault-Llorca F, Rüschoff J, Osamura RY, et al. Value and limitations of measuring HER-2 extracellular domain in the serum of breast cancer patients. J Clin Oncol. 2009. 27:1694–1705.
Article24. Coskun U, Yamac D, Gulbahar O, Sancak B, Karaman N, Ozkan S. Locally advanced breast carcinoma treated with neoadjuvant chemotherapy: are the changes in serum levels of YKL-40, MMP-2 and MMP-9 correlated with tumor response? Neoplasma. 2007. 54:348–352.25. Martínez-Trufero J, de Lobera AR, Lao J, Puértolas T, Artal-Cortés A, Zorrilla M, et al. Serum markers and prognosis in locally advanced breast cancer. Tumori. 2005. 91:522–530.
Article26. Schippinger W, Dandachi N, Regitnig P, Hofmann G, Balic M, Neumann R, et al. The predictive value of EGFR and HER-2/neu in tumor tissue and serum for response to anthracycline-based neoadjuvant chemotherapy of breast cancer. Am J Clin Pathol. 2007. 128:630–637.
Article27. Nolen BM, Marks JR, Ta'san S, Rand A, Luong TM, Wang Y, et al. Serum biomarker profiles and response to neoadjuvant chemotherapy for locally advanced breast cancer. Breast Cancer Res. 2008. 10:R45.
Article28. Tiezzi DG, Andrade JM, Ribeiro-Silva A, Zola FE, Marana HR, Tiezzi MG. HER-2, p53, p21 and hormonal receptors proteins expression as predictive factors of response and prognosis in locally advanced breast cancer treated with neoadjuvant docetaxel plus epirubicin combination. BMC Cancer. 2007. 7:36.
Article29. Witzel I, Loibl S, von Minckwitz G, Mundhenke C, Huober J, Hanusch C, et al. Monitoring serum HER2 levels during neoadjuvant trastuzumab treatment within the GeparQuattro trial. Breast Cancer Res Treat. 2010. 123:437–445.
Article30. Leyland-Jones B, Smith BR. Serum HER2 testing in patients with HER2-positive breast cancer: the death knell tolls. Lancet Oncol. 2011. 12:286–295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in Protein Expression in Breast Cancer after Anthracycline-Based Chemotherapy
- Ki-67 as a Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients
- Serum HER2 as a Response Indicator to Various Chemotherapeutic Agents in Tissue HER2 Positive Metastatic Breast Cancer
- Impact of Immunohistochemistry-Based Molecular Subtype on Chemosensitivity and Survival in Patients with Breast Cancer Following Neoadjuvant Chemotherapy
- Prognostic Value of the Evolution of HER2-Low Expression after Neoadjuvant Chemotherapy