J Bone Metab.
2014 May;21(2):85-98. 10.11005/jbm.2014.21.2.85.
Pathobiology of Paget's Disease of Bone
- Affiliations
-
- 1Department of Medicine/Hematology-Oncology, University of Pittsburgh Cancer Institute, University of Pittsburgh School of Medicine, Pittsburgh PA, USA.
- 2Department of Medicine/Hematology-Oncology, Indiana University, Indianapolis IN, USA. groodman@iu.edu
- 3Veterans Administration Medical Center, Indianapolis, IN, USA.
- KMID: 2241530
- DOI: http://doi.org/10.11005/jbm.2014.21.2.85
Abstract
- Paget's disease of bone is characterized by highly localized areas of increased bone resorption accompanied by exuberant, but aberrant new bone formation with the primary cellular abnormality in osteoclasts. Paget's disease provides an important paradigm for understanding the molecular mechanisms regulating both osteoclast formation and osteoclast-induced osteoblast activity. Both genetic and environmental etiologies have been implicated in Paget's disease, but their relative contributions are just beginning to be defined. To date, the only gene with mutations in the coding region linked to Paget's disease is sequestosome-1 (SQSTM1), which encodes the p62 protein, and these mutations lead to elevated cytokine activation of NF-B in osteoclasts but do not induce a "pagetic osteoclast" phenotype. Further, genetic mutations linked to Paget's appear insufficient to cause Paget's disease and additional susceptibility loci or environmental factors may be required. Among the environmental factors suggested to induce Paget's disease, chronic measles (MV) infection has been the most studied. Expression of the measles virus nucleocapsid gene (MVNP) in osteoclasts induces pagetic-like osteoclasts and bone lesions in mice. Further, mice expressing both MVNP in osteoclasts and germline mutant p62 develop dramatic pagetic bone lesions that were strikingly similar to those seen in patients with Paget's disease. Thus, interactions between environmental and genetic factors appear important to the development of Paget's disease. In this article we review the mechanisms responsible for the effects of mutant p62 gene expression and MVNP on osteoclast and osteoblast activity, and how they may contribute to the development of Paget's disease of bone.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Intravenous Zoledronate for a Patient with Paget's Disease
Ki-Choul Kim
J Bone Metab. 2014;21(3):223-226. doi: 10.11005/jbm.2014.21.3.223.Paget's Disease: Skeletal Manifestations and Effect of Bisphosphonates
Ho Kang, Young-Chang Park, Kyu Hyun Yang
J Bone Metab. 2017;24(2):97-103. doi: 10.11005/jbm.2017.24.2.97.
Reference
-
1. Roodman GD, Windle JJ. Paget disease of bone. J Clin Invest. 2005; 115:200–208.
Article2. Teramachi J, Kurihara N, Windle J, et al. Expression of measles virus nucleocapsid protein (MVNP) gene in osteoclasts induces coupling factors that stimulate bone formation. Poster sessions presented at: ASBMR 2012 Annual Meeting. 2012 October 12-15; Minesota, USA.3. Basle M, Minard MF, Rebel A. Structure and ultrastructure of osteoblasts and of the osteoid tissue in Paget's disease of bone (author's transl). Pathol Biol (Paris). 1978; 26:475–479.4. Zajac AJ, Phillips PE. Paget's disease of bone: clinical features and treatment. Clin Exp Rheumatol. 1985; 3:75–88.5. Chung PY, Van Hul W. Paget's disease of bone: evidence for complex pathogenetic interactions. Semin Arthritis Rheum. 2012; 41:619–641.
Article6. Tiegs RD, Lohse CM, Wollan PC, et al. Long-term trends in the incidence of Paget's disease of bone. Bone. 2000; 27:423–427.
Article7. Morales-Piga AA, Rey-Rey JS, Corres-González J, et al. Frequency and characteristics of familial aggregation of Paget's disease of bone. J Bone Miner Res. 1995; 10:663–670.
Article8. Siris ES, Jacobs TP, Canfield RE. Paget's disease of bone. Bull N Y Acad Med. 1980; 56:285–304.9. Roodman GD. Insights into the pathogenesis of Paget's disease. Ann N Y Acad Sci. 2010; 1192:176–180.
Article10. Rogov V, Dotsch V, Johansen T, et al. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell. 2014; 53:167–178.
Article11. Hocking LJ, Lucas GJ, Daroszewska A, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget's disease. Hum Mol Genet. 2002; 11:2735–2739.
Article12. Laurin N, Brown JP, Morissette J, et al. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. 2002; 70:1582–1588.
Article13. Morissette J, Laurin N, Brown JP. Sequestosome 1: mutation frequencies, haplotypes, and phenotypes in familial Paget's disease of bone. J Bone Miner Res. 2006; 21:Suppl 2. P38–P44.
Article14. Bolland MJ, Tong PC, Naot D, et al. Delayed development of Paget's disease in offspring inheriting SQSTM1 mutations. J Bone Miner Res. 2007; 22:411–415.
Article15. Gennari L, Gianfrancesco F, Di Stefano M, et al. SQSTM1 gene analysis and gene-environment interaction in Paget's disease of bone. J Bone Miner Res. 2010; 25:1375–1384.
Article16. Leach RJ, Singer FR, Ench Y, et al. Clinical and cellular phenotypes associated with sequestosome 1 (SQSTM1) mutations. J Bone Miner Res. 2006; 21:Suppl 2. P45–P50.
Article17. Doyle T, Gunn J, Anderson G, et al. Paget's disease in New Zealand: evidence for declining prevalence. Bone. 2002; 31:616–619.
Article18. van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget's disease of bone in England and Wales. J Bone Miner Res. 2002; 17:465–471.
Article19. Poór G, Donath J, Fornet B, et al. Epidemiology of Paget's disease in Europe: the prevalence is decreasing. J Bone Miner Res. 2006; 21:1545–1549.
Article20. Cundy HR, Gamble G, Wattie D, et al. Paget's disease of bone in New Zealand: continued decline in disease severity. Calcif Tissue Int. 2004; 75:358–364.
Article21. Albagha OM, Visconti MR, Alonso N, et al. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget's disease of bone. Nat Genet. 2010; 42:520–524.
Article22. Albagha OM, Wani SE, Visconti MR, et al. Genome-wide association identifies three new susceptibility loci for Paget's disease of bone. Nat Genet. 2011; 43:685–689.
Article23. Chung PY, Beyens G, Boonen S, et al. The majority of the genetic risk for Paget's disease of bone is explained by genetic variants close to the CSF1, OPTN, TM7SF4, and TNFRSF11A genes. Hum Genet. 2010; 128:615–626.
Article24. Chung PY, Beyens G, Riches PL, et al. Genetic variation in the TNFRSF11A gene encoding RANK is associated with susceptibility to Paget's disease of bone. J Bone Miner Res. 2010; 25:2592–2605.
Article25. Daroszewska A, Hocking LJ, McGuigan FE, et al. Susceptibility to Paget's disease of bone is influenced by a common polymorphic variant of osteoprotegerin. J Bone Miner Res. 2004; 19:1506–1511.
Article26. Kukita T, Wada N, Kukita A, et al. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med. 2004; 200:941–946.
Article27. Yagi M, Miyamoto T, Sawatani Y, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005; 202:345–351.
Article28. Kachaner D, Génin P, Laplantine E, et al. Toward an integrative view of Optineurin functions. Cell Cycle. 2012; 11:2808–2818.
Article29. Grandi P, Dang T, Pané N, et al. Nup93, a vertebrate homologue of yeast Nic96p, forms a complex with a novel 205-kDa protein and is required for correct nuclear pore assembly. Mol Biol Cell. 1997; 8:2017–2038.
Article30. Kajiho H, Saito K, Tsujita K, et al. RIN3: a novel Rab5 GEF interacting with amphiphysin II involved in the early endocytic pathway. J Cell Sci. 2003; 116:4159–4168.
Article31. Itzstein C, Coxon FP, Rogers MJ. The regulation of osteoclast function and bone resorption by small GTPases. Small GTPases. 2011; 2:117–130.
Article32. Lin HK, Bergmann S, Pandolfi PP. Cytoplasmic PML function in TGF-beta signalling. Nature. 2004; 431:205–211.33. Smits P, Bolton AD, Funari V, et al. Lethal skeletal dysplasia in mice and humans lacking the golgin GMAP-210. N Engl J Med. 2010; 362:206–216.
Article34. Hennies HC, Kornak U, Zhang H, et al. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat Genet. 2008; 40:1410–1412.
Article35. Ralston SH, Layfield R. Pathogenesis of Paget disease of bone. Calcif Tissue Int. 2012; 91:97–113.
Article36. Rebel A, Malkani K, Baslé M, et al. Is Paget's disease of bone a viral infection? Calcif Tissue Res. 1977; 22:Suppl. 283–286.
Article37. Mills BG, Singer FR, Weiner LP, et al. Evidence for both respiratory syncytial virus and measles virus antigens in the osteoclasts of patients with Paget's disease of bone. Clin Orthop Relat Res. 1984; 303–311.
Article38. Gordon MT, Mee AP, Sharpe PT. Paramyxoviruses in Paget's disease. Semin Arthritis Rheum. 1994; 23:232–234.
Article39. Mee AP, Dixon JA, Hoyland JA, et al. Detection of canine distemper virus in 100% of Paget's disease samples by in situ-reverse transcriptase-polymerase chain reaction. Bone. 1998; 23:171–175.
Article40. Kurihara N, Hiruma Y, Yamana K, et al. Contributions of the measles virus nucleocapsid gene and the SQSTM1/p62 (P392L) mutation to Paget's disease. Cell Metab. 2011; 13:23–34.
Article41. Kurihara N, Reddy SV, Menaa C, et al. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest. 2000; 105:607–614.
Article42. Gordon MT, Mee AP, Anderson DC, et al. Canine distemper virus transcripts sequenced from pagetic bone. Bone Miner. 1992; 19:159–174.
Article43. Reddy SV, Kurihara N, Menaa C, et al. Osteoclasts formed by measles virus-infected osteoclast precursors from hCD46 transgenic mice express characteristics of pagetic osteoclasts. Endocrinology. 2001; 142:2898–2905.
Article44. Kurihara N, Hiruma Y, Zhou H, et al. Mutation of the sequestosome 1 (p62) gene increases osteoclastogenesis but does not induce Paget disease. J Clin Invest. 2007; 117:133–142.
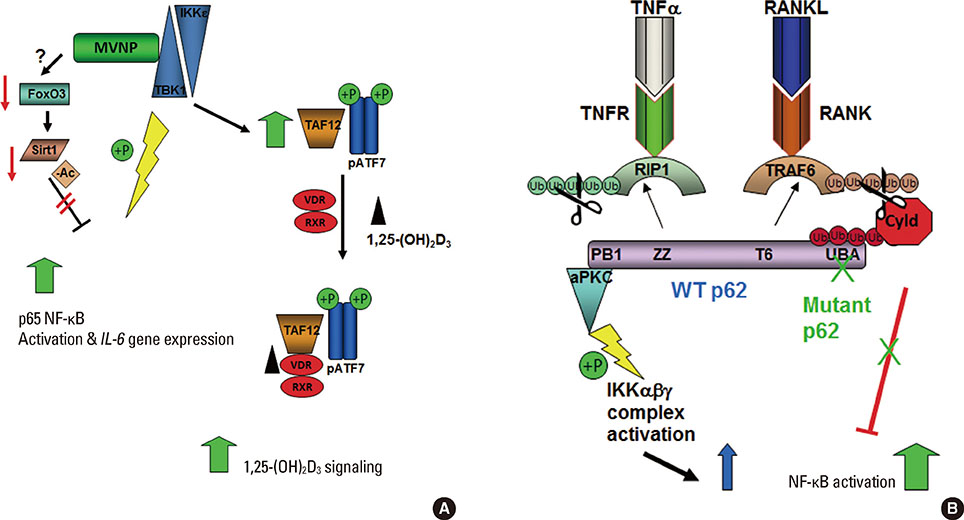
Article45. Daroszewska A, van't Hof RJ, Rojas JA, et al. A point mutation in the ubiquitin-associated domain of SQSMT1 is sufficient to cause a Paget's disease-like disorder in mice. Hum Mol Genet. 2011; 20:2734–2744.
Article46. Kurihara N, Zhou H, Reddy SV, et al. Expression of measles virus nucleocapsid protein in osteoclasts induces Paget's disease-like bone lesions in mice. J Bone Miner Res. 2006; 21:446–455.
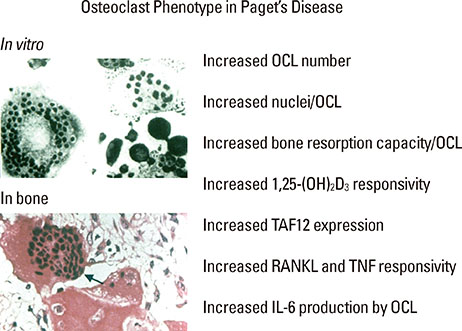
Article47. Roodman GD. Interleukin-6: an osteotropic factor? J Bone Miner Res. 1992; 7:475–478.48. Menaa C, Reddy SV, Kurihara N, et al. Enhanced RANK ligand expression and responsivity of bone marrow cells in Paget's disease of bone. J Clin Invest. 2000; 105:1833–1838.
Article49. Teramachi J, Zhou H, Subler MA, et al. Increased IL-6 Expression in Osteoclasts is Necessary but not Sufficient for the Development of Paget's Disease of Bone. J Bone Miner Res. 2013.
Article50. Neale SD, Smith R, Wass JA, et al. Osteoclast differentiation from circulating mononuclear precursors in Paget's disease is hypersensitive to 1,25-dihydroxyvitamin D(3) and RANKL. Bone. 2000; 27:409–416.
Article51. Mengus G, Gangloff YG, Carré L, et al. The human transcription factor IID subunit human TATA-binding protein-associated factor 28 interacts in a ligand-reversible manner with the vitamin D(3) and thyroid hormone receptors. J Biol Chem. 2000; 275:10064–10071.
Article52. Hamard PJ, Dalbies-Tran R, Hauss C, et al. A functional interaction between ATF7 and TAF12 that is modulated by TAF4. Oncogene. 2005; 24:3472–3483.
Article53. Kurihara N, Reddy SV, Araki N, et al. Role of TAFII-17, a VDR binding protein, in the increased osteoclast formation in Paget's Disease. J Bone Miner Res. 2004; 19:1154–1164.
Article54. Teramachi J, Hiruma Y, Ishizuka S, et al. Role of ATF7-TAF12 interactions in the vitamin D response hypersensitivity of osteoclast precursors in Paget's disease. J Bone Miner Res. 2013; 28:1489–1500.
Article55. Ye H, Arron JR, Lamothe B, et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002; 418:443–447.
Article56. Besse A, Lamothe B, Campos AD, et al. TAK1-dependent signaling requires functional interaction with TAB2/TAB3. J Biol Chem. 2007; 282:3918–3928.
Article57. Durán A, Serrano M, Leitges M, et al. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell. 2004; 6:303–309.
Article58. Chamoux E, McManus S, Laberge G, et al. Involvement of kinase PKC-zeta in the p62/p62(P392L)-driven activation of NF-kappaB in human osteoclasts. Biochim Biophys Acta. 2013; 1832:475–484.
Article59. Sanz L, Sanchez P, Lallena MJ, et al. The interaction of p62 with RIP links the atypical PKCs to NF-kappaB activation. EMBO J. 1999; 18:3044–3053.
Article60. Sundaram K, Shanmugarajan S, Rao DS, et al. Mutant p62P392L stimulation of osteoclast differentiation in Paget's disease of bone. Endocrinology. 2011; 152:4180–4189.
Article61. Nagabhushana A, Bansal M, Swarup G. Optineurin is required for CYLD-dependent inhibition of TNFalpha-induced NF-kappaB activation. PLoS One. 2011; 6:e17477.62. Wang FM, Sarmasik A, Hiruma Y, et al. Measles virus nucleocapsid protein, a key contributor to Paget's disease, increases IL-6 expression via down-regulation of FoxO3/Sirt1 signaling. Bone. 2013; 53:269–276.
Article63. Keller ET, Chang C, Ershler WB. Inhibition of NFkappaB activity through maintenance of IkappaBalpha levels contributes to dihydrotestosterone-mediated repression of the interleukin-6 promoter. J Biol Chem. 1996; 271:26267–26275.
Article64. Hu HM, Tian Q, Baer M, et al. The C/EBP bZIP domain can mediate lipopolysaccharide induction of the proinflammatory cytokines interleukin-6 and monocyte chemoattractant protein-1. J Biol Chem. 2000; 275:16373–16381.
Article65. Sharma S, tenOever BR, Grandvaux N, et al. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003; 300:1148–1151.
Article66. tenOever BR, Servant MJ, Grandvaux N, et al. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. J Virol. 2002; 76:3659–3669.
Article67. Nagy ZB, Gergely P, Donáth J, et al. Gene expression profiling in Paget's disease of bone: upregulation of interferon signaling pathways in pagetic monocytes and lymphocytes. J Bone Miner Res. 2008; 23:253–259.
Article68. Adli M, Baldwin AS. IKK-i/IKKepsilon controls constitutive, cancer cell-associated NF-kappaB activity via regulation of Ser-536 p65/RelA phosphorylation. J Biol Chem. 2006; 281:26976–26984.
Article69. Buss H, Dorrie A, Schmitz ML, et al. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-{kappa}B at serine 536 is mediated by multiple protein kinases including I{kappa}B kinase (IKK)-{alpha}, IKK{beta}, IKK{epsilon}, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem. 2004; 279:55633–55643.
Article70. Sun Q, Sammut B, Wang FM, et al. TBK1 mediates critical effects of measles virus nucleocapsid protein (MVNP) on pagetic osteoclast formation. J Bone Miner Res. 2014; 29:90–102.
Article71. Greene WC, Chen LF. Regulation of NF-kappaB action by reversible acetylation. Novartis Found Symp. 2004; 259:208–217. discussion 18-25.72. Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999; 96:857–868.
Article73. Luron L, Saliba D, Blazek K, et al. FOXO3 as a new IKK-epsilon-controlled check-point of regulation of IFN-beta expression. Eur J Immunol. 2012; 42:1030–1037.
Article74. Yang JY, Zong CS, Xia W, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008; 10:138–148.
Article75. Ishizuka H, García-Palacios V, Lu G, et al. ADAM8 enhances osteoclast precursor fusion and osteoclast formation in vitro and in vivo. J Bone Miner Res. 2011; 26:169–181.
Article76. Xing L, Xiu Y, Boyce BF. Osteoclast fusion and regulation by RANKL-dependent and independent factors. World J Orthop. 2012; 3:212–222.
Article77. Sarmasik A, Hiruma Y, Okumura S, et al. Measles virus nucleoprotein (MVNP) enhances NFATcl activation during osteoclastogenesis in Paget's disease. J Bone Miner Res. 2007; 22:Suppl 1. S221.78. Kim K, Lee SH, Ha Kim J, et al. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol Endocrinol. 2008; 22:176–185.
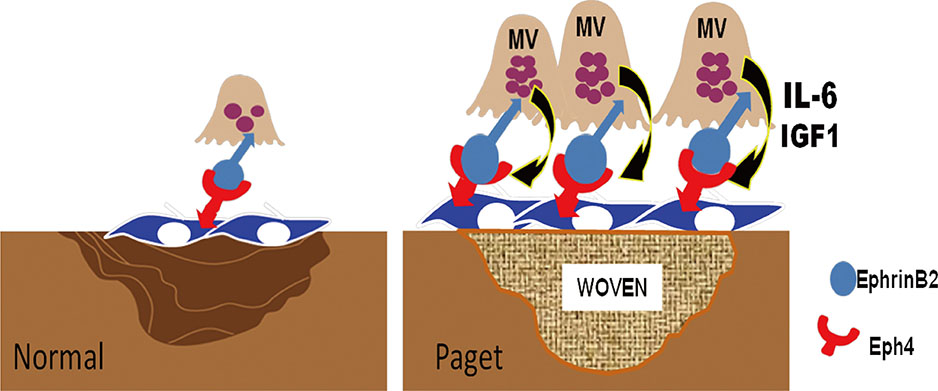
Article79. Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adh Migr. 2012; 6:148–156.80. Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006; 4:111–121.
Article81. Hayashi M, Nakashima T, Taniguchi M, et al. Osteoprotection by semaphorin 3A. Nature. 2012; 485:69–74.
Article82. Zhang M, Xuan S, Bouxsein ML, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002; 277:44005–44012.
Article83. Kurihara N, Teramachi J, Kitagawa Y, et al. IGF1 contributes to the increased bone formation induced by measles virus nucleocapsid protein expressed by osteoclasts in Paget's bone disease. Poster sessions presented at: ASBMR 2013 Annual Meeting. 2013 October 4-7; Baltimore, MD, USA.84. Sundaram K, Senn J, Yuvaraj S, et al. FGF-2 stimulation of RANK ligand expression in Paget's disease of bone. Mol Endocrinol. 2009; 23:1445–1454.
Article85. Zhang X, Sobue T, Hurley MM. FGF-2 increases colony formation, PTH receptor, and IGF-1 mRNA in mouse marrow stromal cells. Biochem Biophys Res Commun. 2002; 290:526–531.
Article86. Sundaram K, Senn J, Reddy SV. SOCS-1/3 participation in FGF-2 signaling to modulate RANK ligand expression in paget's disease of bone. J Cell Biochem. 2013; 114:2032–2038.
Article