Korean J Lab Med.
2006 Aug;26(4):307-315. 10.3343/kjlm.2006.26.4.307.
Quality Evaluation of the Performance Study of Diagnostic Tests Using STARD Checklist and Meta-Analysis for the Pooled Sensitivity and Specificity of Third Generation Anti-HCV EIA Tests
- Affiliations
-
- 1Department of Laboratory Medicine, Asan Medical Center and University of Ulsan College of Medicine, Seoul, Korea. hboh@amc.seoul.kr
- 2Department of Statistics, Dongguk University, Seoul, Korea.
- KMID: 2238840
- DOI: http://doi.org/10.3343/kjlm.2006.26.4.307
Abstract
-
BACKGROUND: The third generation anti-hepatitis C virus (HCV) enzyme immunoassay (EIA) is now in use for screening HCV infection. The aim of this study was to pool the data on the sensitivity and specificity of third generation anti-HCV EIA tests after evaluating the quality of the studies using Standards for Reporting of Diagnostic Accuracy studies (STARD) checklist.
METHODS
We searched MEDLINE and PubMed databases using keywords about the accuracy of diagnostic tests for HCV infections. Methodological quality was assessed by two persons with a modified STARD checklist. A heterogeneity test was performed, and in case heterogeneity was present, a sub-group analysis was done. Fixed-effects model was used to obtain pool sensitivity and specificity with 95% confidence intervals (CI).
RESULTS
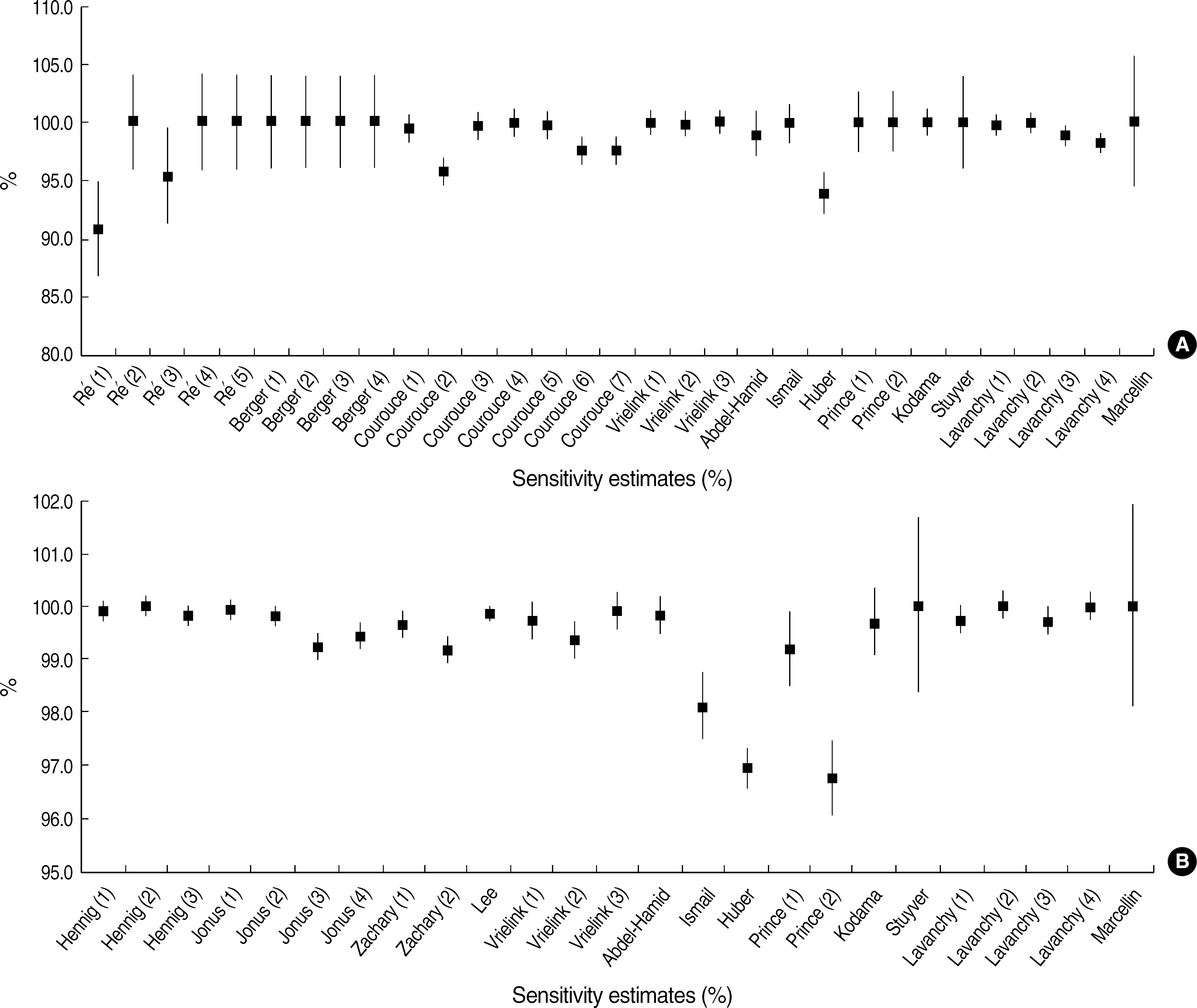
A total of 41 studies from 16 papers were selected. The quality score ranged from 6 to 13 (median 10.5); Inter-observer agreement was 93.62% (k=0.69); and 41 studies revealed heterogeneity. We performed a sub-group analysis with only 28 studies from 13 papers that were evaluated to be of high quality. A subgroup using polymerase chain reaction as the reference test revealed homogeneity and was calculated the pooled sensitivity and specificity of 99.92% (CI 99.77-100.07%) and 99.66% (CI 99.45-99.86%) respectively. Studies on test kits with an increased reactivity to the core region also showed homogeneity in sensitivity and the pooled sensitivity was 99.78% (CI 99.53-100.03%).
CONCLUSIONS
For the first time in Korea, the diagnostic accuracy of test kits was evaluated by meta-analysis using STARD checklist. The methodology shown in this study should help extending laboratory medicine to an evidence-based medicine.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005; 5:558–67.
Article2. Suh DJ, Jeong SH. Current status of hepatitis C virus infection in Korea. Intervirology. 2006; 49:70–5.
Article3. Oh HB, Hwang YS, Cho YJ, Kim DS, Kim SI. Experience of anti-HCV antibody immunoblot test in Korean blood donors. Korean J Blood Transfus. 1997; 8:1–8.4. Wong T, Lee SS. Hepatitis C: a review for primary care physicians. CMAJ. 2006; 174:649–59.
Article5. Colin C, Lanoir D, Touzet S, Meyaud-Kraemer L, Bailly F, Trepo C. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat. 2001; 8:87–95.
Article6. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin Chem. 2003; 49:1–6.7. Re V, Gallego S, Trevino E, Barbas G, Dominguez C, Elbarcha O, et al. Evaluation of five screening tests licensed in Argentina for detection of hepatitis C virus antibodies. Mem Inst Oswaldo Cruz. 2005; 100:303–7.8. Berger A, Doerr HW, Preiser W, Weber B. Lack of correlation between different hepatitis C virus screening and confirmatory assays. J Virol Methods. 1996; 59:141–6.
Article9. Courouce AM, Barin F, Botte C, Lunel F, Maisonneuve P, Maniez M, et al. A comparative evaluation of the sensitivity of seven anti-hepatitis C virus screening tests. Vox Sang. 1995; 69:213–6.10. Hennig H, Schlenke P, Kirchner H, Bauer I, Schulte-Kellinghaus B, Bludau H. Evaluation of newly developed microparticle enzyme immunoassays for the detection of HCV antibodies. J Virol Methods. 2000; 84:181–90.
Article11. Jonas G, Pelzer C, Beckert C, Hausmann M, Kapprell HP. Performance characteristics of the ARCHITECT anti-HCV assay. J Clin Virol. 2005; 34:97–103.
Article12. Zachary P, Ullmann M, Djeddi S, Wendling MJ, Schvoerer E, Stoll-Keller F, et al. Evaluation of two commercial enzyme immunoassays for diagnosis of hepatitis C in the conditions of a virology laboratory. Pathol Biol (Paris). 2004; 52:511–6.13. Lee SR, Wood CL, Lane MJ, Francis B, Gust C, Higgs CM, et al. Increased detection of hepatitis C virus infection in commercial plasma donors by a third-generation screening assay. Transfusion. 1995; 35:845–9.
Article14. Vrielink H, Zaaijer HL, Reesink HW, van der Poel CL, Cuypers HT, Lelie PN. Sensitivity and specificity of three third-generation anti-hepatitis C virus ELISAs. Vox Sang. 1995; 69:14–7.
Article15. Abdel-Hamid M, El-Daly M, El-Kafrawy S, Mikhail N, Strickland GT, Fix AD. Comparison of second- and third-generation enzyme immunoassays for detecting antibodies to hepatitis C virus. J Clin Microbiol. 2002; 40:1656–9.
Article16. Ismail N, Fish GE, Smith MB. Laboratory evaluation of a fully automated chemiluminescence immunoassay for rapid detection of HBsAg, antibodies to HBsAg, and antibodies to hepatitis C virus. J Clin Microbiol. 2004; 42:610–7.
Article17. Huber KR, Sebesta C, Bauer K. Detection of common hepatitis C virus subtypes with a third-generation enzyme immunoassay. Hepatology. 1996; 24:471–3.18. Prince AM, Scheffel JW, Moore B. A search for hepatitis C virus polymerase chain reaction-positive but seronegative subjects among blood donors with elevated alanine aminotransferase. Transfusion. 1997; 37:211–4.
Article19. Kodama T, Ichiyama S, Sato K, Nada T, Nakashima N. Evaluation of a membrane filter assay system, Ortho HCV Ab Quick Pack, for detection of anti-hepatitis C virus antibody. J Clin Microbiol. 1998; 36:1439–40.20. Stuyver L, Claeys H, Wyseur A, Van Arnhem W, De Beenhouwer H, Uytendaele S, et al. Hepatitis C virus in a hemodialysis unit: molecular evidence for nosocomial transmission. Kidney Int. 1996; 49:889–95.
Article21. Lavanchy D, Steinmann J, Moritz A, Frei PC. Evaluation of a new automated third-generation anti-HCV enzyme immunoassay. J Clin Lab Anal. 1996; 10:269–76.
Article22. Marcellin P, Martinot-Peignoux M, Gabriel F, Branger M, Degott C, Elias A, et al. Chronic non-B, non-C hepatitis among blood donors assessed with HCV third generation tests and polymerase chain reaction. J Hepatol. 1993; 19:167–70.
Article23. Deville WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002; 2:9.
Article24. Smidt N, Rutjes AW, van der Windt DA, Ostelo RW, Bossuyt PM, Reitsma JB, et al. Reproducibility of the STARD checklist: an instrument to assess the quality of reporting of diagnostic accuracy studies. BMC Med Res Methodol. 2006; 6:12.
Article25. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006; 295:676–80.
Article26. Irwig L, Macaskill P, Glasziou P, Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol. 1995; 48:119–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Meta-Analysis for the Pooled Sensitivity and Specificity of anti-Human Immunodeficiency Virus Ab Rapid Tests
- Meta-Analysis for the Pooled Sensitivity and Specificity of Hepatitis B Surface Antigen Rapid Tests
- Evaluation of the Efficiency of HCV RT-PCR Using Narrowly Spaced Primers
- An Evaluation of DONG-A HCV 3.2 and Genedia HCV ELISA 3.0 Detecting HCV Antibody
- Evaluation of Jnnotest HCV 3.0, Genedia HCV 3.0 Enzyme Immunoassay Reagents for Hepatitis C Virus Antibody Detection