Korean Diabetes J.
2010 Dec;34(6):331-337. 10.4093/kdj.2010.34.6.331.
Triple Combination Therapy Using Metformin, Thiazolidinedione, and a GLP-1 Analog or DPP-IV Inhibitor in Patients with Type 2 Diabetes Mellitus
- Affiliations
-
- 1Diabetes Center, Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. sunwoo4012.kim@samsung.com
- KMID: 2222359
- DOI: http://doi.org/10.4093/kdj.2010.34.6.331
Abstract
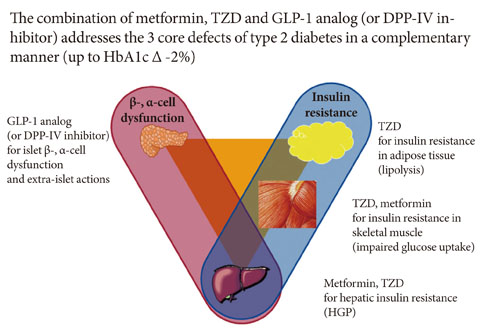
- Although there is no HbA1c threshold for cardiovascular risk, the American Diabetic Association-recommended goal of HbA1c < 7.0% appears to be unacceptably high. To achieve an optimal HbA1c level goal of 6.0% or less, a high dosage of sulfonylureas and insulin would be required; the trade-off would be the common adverse effects of hypoglycemia and weight gain. In contrast, hypoglycemia is uncommon with insulin sensitizers and GLP-1 analogs, allowing the physician to titrate these drugs to maximum dosage to reduce HbA1c levels below 6.0% and they have been shown to preserve beta-cell function. Lastly, weight gain is common with sulfonylurea and insulin therapy, whereas GLP-1 analogs induce weight loss and offset the weight gain associated with TZDs. A treatment paradigm shift is recommended in which combination therapy is initiated with diet/exercise, metformin (which has antiatherogenic effects and improves hepatic insulin sensitivity), a TZD (which improves insulin sensitivity and preserves beta-cell function with proven durability), and a GLP-1 analog (which improves beta, alpha-cell function and promotes weight loss) or a dipeptidyl peptidase IV inhibitor in patients with type 2 diabetes mellitus.
MeSH Terms
Figure
Reference
-
1. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.2. Evans JM, Ogston SA, Emslie-Smith A, Morris AD. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia. 2006. 49:930–936.3. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998. 352:854–865.4. Diamanti-Kandarakis E, Christakou CD, Kandaraki E, Economou FN. Metformin: an old medication of new fashion: evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur J Endocrinol. 2010. 162:193–212.5. Buchanan TA, Xiang AH. Diabetes: preventing type 2 diabetes mellitus: is metformin the answer? Nat Rev Endocrinol. 2010. 6:253–254.6. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006. 355:2427–2443.7. Hanefeld M, Pfutzner A, Forst T, Lubben G. Glycemic control and treatment failure with pioglitazone versus glibenclamide in type 2 diabetes mellitus: a 42-month, open-label, observational, primary care study. Curr Med Res Opin. 2006. 22:1211–1215.8. Home PD, Jones NP, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Komajda M, Curtis P. RECORD Study Group. Rosiglitazone RECORD study: glucose control outcomes at 18 months. Diabet Med. 2007. 24:626–634.9. Mudaliar S, Henry RR. Effects of incretin hormones on beta-cell mass and function, body weight, and hepatic and myocardial function. Am J Med. 2010. 123:3 Suppl. S19–S27.10. Bunck MC, Diamant M, Corner A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Yki-Jarvinen H, Heine RJ. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009. 32:762–768.11. Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Hol-combe JH, Wintle ME, Maggs DG. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008. 24:275–286.12. Menge BA, Tannapfel A, Belyaev O, Drescher R, Muller C, Uhl W, Schmidt WE, Meier JJ. Partial pancreatectomy in adult humans does not provoke beta-cell regeneration. Diabetes. 2008. 57:142–149.13. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006. 3:153–165.14. Ahren B, Pacini G, Foley JE, Schweizer A. Improved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 year. Diabetes Care. 2005. 28:1936–1940.15. Deacon CF. Dipeptidyl peptidase 4 inhibition with sitagliptin: a new therapy for type 2 diabetes. Expert Opin Investig Drugs. 2007. 16:533–545.16. Balas B, Baig MR, Watson C, Dunning BE, Ligueros-Saylan M, Wang Y, He YL, Darland C, Holst JJ, Deacon CF, Cusi K, Mari A, Foley JE, DeFronzo RA. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007. 92:1249–1255.17. Tomas E, Habener JF. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends Endocrinol Metab. 2010. 21:59–67.18. Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006. 28:1556–1568.19. Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007. 9:166–174.20. Rosenstock J, Kim SW, Baron MA, Camisasca RP, Cressier F, Couturier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007. 9:175–185.21. Pratley RE, Reusch JE, Fleck PR, Wilson CA, Mekki Q. Alogliptin Study 009 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin. 2009. 25:2361–2371.22. Gupta D, Peshavaria M, Monga N, Jetton TL, Leahy JL. Physiologic and pharmacologic modulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in beta-cells by peroxisome proliferator-activated receptor (PPAR)-gamma signaling: possible mechanism for the GIP resistance in type 2 diabetes. Diabetes. 2010. 59:1445–1450.23. Solomon TP, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care. 2010. 33:1561–1566.24. Pratley RE. GIP: an inconsequential incretin or not? Diabetes Care. 2010. 33:1691–1692.25. Zinman B, Hoogwerf BJ, Duran Garcia S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007. 146:477–485.26. Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L. LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009. 32:1224–1230.27. Buse JB, Sesti G, Schmidt WE, Montanya E, Chang CT, Xu Y, Blonde L, Rosenstock J. Liraglutide Effect Action in Diabetes-6 Study Group. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010. 33:1300–1303.28. DeFronzo RA, Triplitt C, Qu Y, Lewis MS, Maggs D, Glass LC. Effects of exenatide plus rosiglitazone on beta-cell function and insulin sensitivity in subjects with type 2 diabetes on metformin. Diabetes Care. 2010. 33:951–957.29. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009. 58:773–795.30. Chia CW, Egan JM. Incretin-based therapies in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2008. 93:3703–3716.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DPP-4 Inhibitors as a New Option for the Management of Type 2 Diabetes
- Clinical Characteristics and Metabolic Predictors of Rapid Responders to Dipeptidyl Peptidase-4 Inhibitor as an Add-on Therapy to Sulfonylurea and Metformin
- Antihyperglycemic agent combination therapy for patients with type 2 diabetes mellitus
- Sodium Glucose Cotransporter-2 Inhibitors as an Add-on Therapy to Metformin Plus Dipeptidyl Peptidase-4 Inhibitor in Patients with Type 2 Diabetes
- Diabetes Medications and Cardiovascular Disease Prevention