J Periodontal Implant Sci.
2010 Jun;40(3):111-118. 10.5051/jpis.2010.40.3.111.
Evaluation of vitrification for cryopreservation of teeth
- Affiliations
-
- 1Oral Cancer Research Institute, Department of Oral Pathology, Yonsei University College of Dentistry, Seoul, Korea. jink@yuhs.ac
- 2Department of Periodontology, Yonsei University College of Dentistry, Seoul, Korea.
- 3Department of Conservative Dentistry, Yonsei University College of Dentistry, Seoul, Korea.
- KMID: 2212137
- DOI: http://doi.org/10.5051/jpis.2010.40.3.111
Abstract
- PURPOSE
The aim of this study was to investigate whether vitrification in the cryopreservation of periodontal ligament (PDL) cells could be useful for tooth banking.
METHODS
In step 1, primary cultured human PDL cells were cryopreserved in 100% conventional cryopreservation media and 100% vitrification media (ESF40 media) in different temperatures for 2 weeks. In step 2, a series of modified vitrification formulae named T1 (75% vitrification media + 25% F media), T2 (50% vitrification media + 50% F media) and T3 (25% vitrification media + 75% F media) were used to store PDL cells for 2 weeks and 4 weeks in liquid nitrogen. MTT assay was performed to examine the viability of PDL cells.
RESULTS
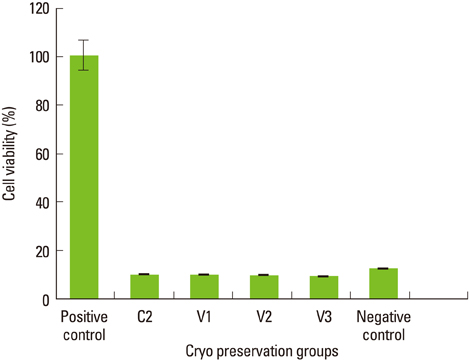
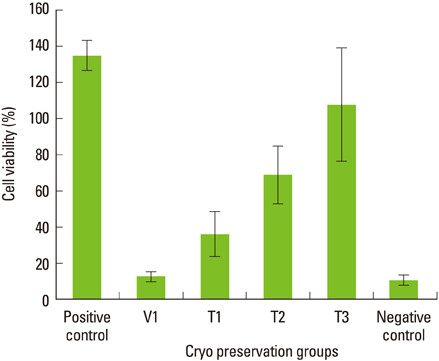
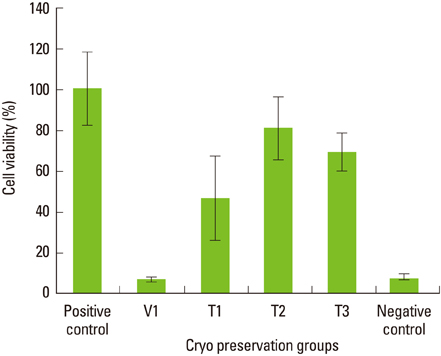
Maximum cell viability was achieved in cells stored in 100% conventional cryopreservation media at -196degrees C (positive control group) in step 1. Compared to the positive control group, viability of the cells stored in 100% vitrification media was very low as 10% in all test conditions. In step 2, as the percentage of vitrification media decreased, the cell viability increased in cells stored for 2 weeks. In 4-week storage of cells in step 2, higher cell viability was observed in the T2 group than the other vitrification formulae while the positive control group had the highest viability. There was no statistically significant difference in the cell viability of 2-week and 4-week stored cells in the T2 group.
CONCLUSIONS
These observations indicate 100% vitrification media is not successful in PDL cell cryopreservation. Conventional cryopreservation media is currently the most appropriate media type for this purpose while T2 media would be interesting to test for long-term storage of PDL cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Kristerson L, Johansson LA, Kisch J, Stadler LE. Autotransplantation of third molars as treatment in advanced periodontal disease. J Clin Periodontol. 1991. 18:521–528.
Article2. Schwartz O, Andreasen JO, Greve T. Cryopreservation before replantation of mature teeth in monkeys. (II). Effect of preincubation, different freezing and equilibration rates and endodontic treatment upon periodontal healing. Int J Oral Surg. 1985. 14:350–361.3. Oh YH, Che ZM, Hong JC, Lee EJ, Lee SJ, Kim J. Cryopreservation of human teeth for future organization of a tooth bank: a preliminary study. Cryobiology. 2005. 51:322–329.
Article4. Temmerman L, De Pauw GA, Beele H, Dermaut LR. Tooth transplantation and cryopreservation: state of the art. Am J Orthod Dentofacial Orthop. 2006. 129:691–695.
Article5. Temmerman L, Dermaut LR, De Mil M, Van Maele G, Beele H, De Pauw GA. Influence of cryopreservation on human periodontal ligament cells in vitro. Cell Tissue Bank. 2008. 9:11–18.
Article6. Kasai M, Mukaida T. Cryopreservation of animal and human embryos by vitrification. Reprod Biomed Online. 2004. 9:164–170.
Article7. Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985. 313:573–575.
Article8. Takahashi T, Hirsh A, Erbe EF, Bross JB, Steere RL, Williams RJ. Vitrification of human monocytes. Cryobiology. 1986. 23:103–115.
Article9. Decherchi P, Lammari-Barreault N, Cochard P, Carin M, Rega P, Pio J, et al. CNS axonal regeneration with peripheral nerve grafts cryopreserved by vitrification: cytological and functional aspects. Cryobiology. 1997. 34:214–239.
Article10. Song YC, Khirabadi BS, Lightfoot F, Brockbank KG, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nat Biotechnol. 2000. 18:296–299.
Article11. Pegg DE. The role of vitrification techniques of cryopreservation in reproductive medicine. Hum Fertil (Camb). 2005. 8:231–239.
Article12. Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984. 21:407–426.
Article13. Kasai M, Komi JH, Takakamo A, Tsudera H, Sakurai T, Machida T. A simple method for mouse embryo cryopreservation in a low toxicity vitrification solution, without appreciable loss of viability. J Reprod Fertil. 1990. 89:91–97.
Article14. Pegg DE. The history and principles of cryopreservation. Semin Reprod Med. 2002. 20:5–13.
Article15. Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, et al. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction. 2009. 138:319–327.
Article16. Armitage WJ. Survival of corneal endothelium following exposure to a vitrification solution. Cryobiology. 1989. 26:318–327.
Article17. Bourne WM, Nelson LR. Human corneal studies with a vitrification solution containing dimethyl sulfoxide, formamide, and 1,2-propanediol. Cryobiology. 1994. 31:522–530.
Article18. Armitage WJ, Hall SC, Routledge C. Recovery of endothelial function after vitrification of cornea at -110 degrees C. Invest Ophthalmol Vis Sci. 2002. 43:2160–2164.19. Makarevich AV, Chrenek P, Olexikova L, Popelkova M, Turanova Z, Ostro A, et al. Post-thaw survival, cell death and actin cytoskeleton in gene-microinjected rabbit embryos after vitrification. Theriogenology. 2008. 70:675–681.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Slush-nitrogen on the Cryopreservation of Oocytes and Embryos using Vitrification
- Comparison of In Vitro Development of Mouse Early Blastocysts according to Vitrification and Conventional Slow Freezing Procedures
- Cryopreservation of Oocytes and Embryos by Vitrification
- Comparison of Vitrification and Slow Freezing for the Cryopreservation of Mouse Pronuclear Stage Embryos
- Vitrification and Ultrarapid Freezing of Day 2 Mouse Embryos