J Korean Surg Soc.
2010 Jun;78(6):376-384. 10.4174/jkss.2010.78.6.376.
The 10 Years of Experiences with GISTs
- Affiliations
-
- 1Department of Surgery, Research Institute for Medical Science, College of Medicine, Chungnam National University, Daejeon, Korea. Songis@cnu.ac.kr
- 2Department of Pathology, Research Institute for Medical Science, College of Medicine, Chungnam National University, Daejeon, Korea.
- KMID: 2211968
- DOI: http://doi.org/10.4174/jkss.2010.78.6.376
Abstract
- PURPOSE
Gastrointestinal stromal tumors (GISTs) possess highly variable clinical behaviors and the study thereof is insufficient. There are no standard guidelines for diagnosis and treatment of GISTs, so it is difficult to predict recurrences and conduct appropriate treatments. Throughout the last 10 years of experiences with GIST patients, we have identified the variables predicting recurrences and classified the risk groups by NIH classification, Fletcher risk stratification and UICC TNM stage.
METHODS
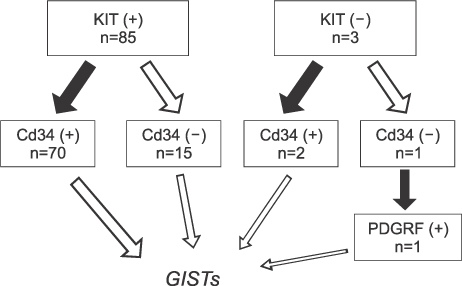
From January 1998 to December 2007, 88 patients with pathologic confirm and surgical resection were diagnosed with GISTs. GISTs were diagnosed when the tumor had characteristic histologic features and confirmed positive by KIT, CD34, or PDGFRA.
RESULTS
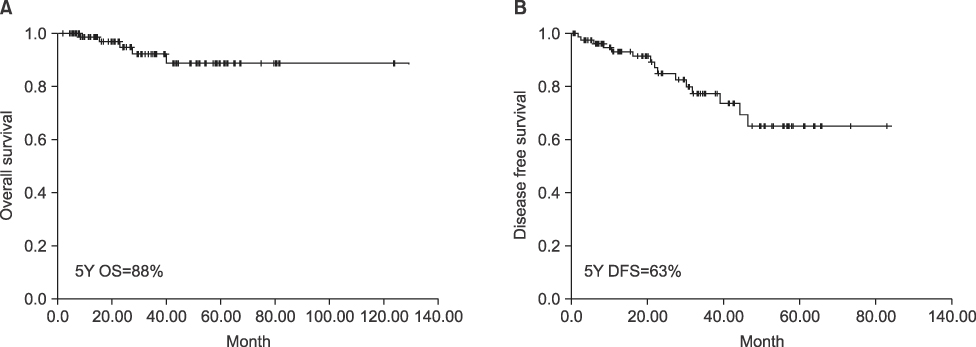
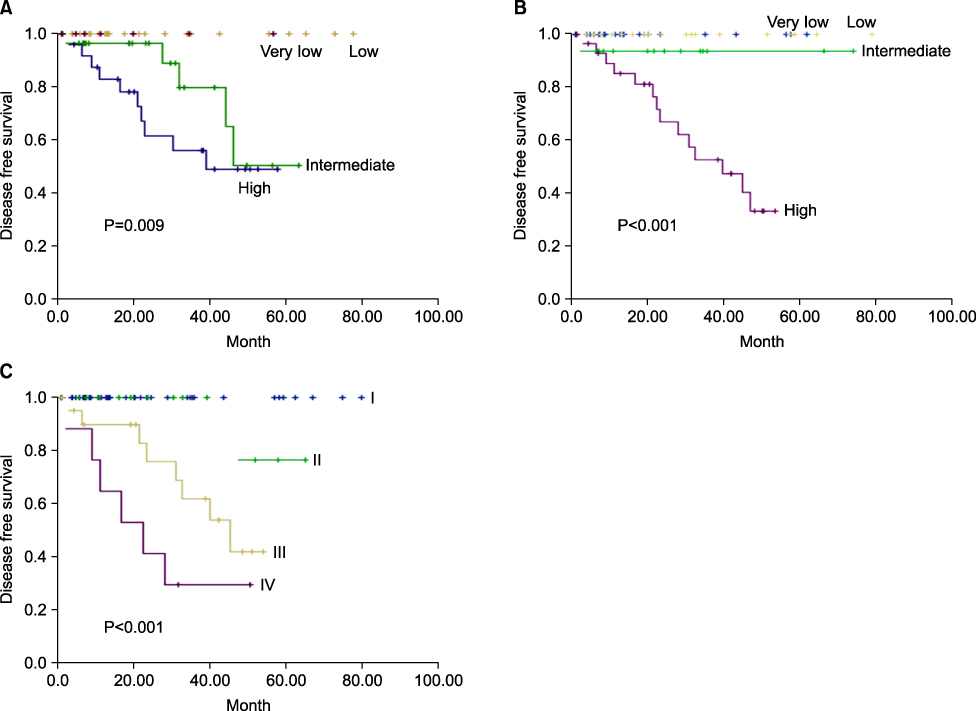
The size, mitotic index, existence of symptoms, and origins of tumor correlate statistically with recurrence (0.002, <0.001, 0.027, 0.011). The NIH classification, Fletcher risk stratification and UICC TNM stage are correlated with recurrence (0.001, <0.001, <0.001) and 5 year disease free survival, statistically (0.009, <0.001, <0.001). Fifteen patients experienced recurrences. 14 patients were treated with imatinib, and 6 of them showed a response to the treatment. All 4 patients who had R1 resection did not survived due to the progression of the disease.
CONCLUSION
The patients with large, high mitotic index, symptomatic, or extra-gastric tumor require strict surveillance. Also, patients with low risk must be under surveillance due to the possibility of recurrence. It is important to perform R0, en bloc resection. Although the imatinib is the treatment of choice with recurred or metastatic GISTs, the disease is likely to develop resistance, further studies on newly targeted therapy is in need.
Keyword
MeSH Terms
Figure
Reference
-
1. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002. 33:459–465.2. Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998. 152:1259–1269.3. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998. 279:577–580.4. Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003. 299:708–710.5. Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002. 160:1567–1572.6. Amin MB, Ma CK, Linden MD, Kubus JJ, Zarbo RJ. Prognostic value of proliferating cell nuclear antigen index in gastric stromal tumors. Correlation with mitotic count and clinical outcome. Am J Clin Pathol. 1993. 100:428–432.7. Hornick JL, Fletcher CD. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007. 38:679–687.8. Rubin BP. Gastrointestinal stromal tumours: an update. Histopathology. 2006. 48:83–96.9. Bauer S, Corless CL, Heinrich MC, Dirsch O, Antoch G, Kanja J, et al. Response to imatinib mesylate of a gastrointestinal stromal tumor with very low expression of KIT. Cancer Chemother Pharmacol. 2003. 51:261–265.10. Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002. 33:478–483.11. Mochizuki Y, Kodera Y, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, et al. Treatment and risk factors for recurrence after curative resection of gastrointestinal stromal tumors of the stomach. World J Surg. 2004. 28:870–875.12. Samiian L, Weaver M, Velanovich V. Evaluation of gastrointestinal stromal tumors for recurrence rates and patterns of long-term follow-up. Am Surg. 2004. 70:187–191.13. Langer C, Gunawan B, Schuler P, Huber W, Fuzesi L, Becker H. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg. 2003. 90:332–339.14. van der Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor: 5 years later. Cancer. 2005. 104:1781–1788.15. Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005. 16:566–578.16. Emory TS, Sobin LH, Lukes L, Lee DH, O'Leary TJ. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999. 23:82–87.17. Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005. 29:1373–1381.18. Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000. 295:139–145.19. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002. 347:472–480.20. Demetri GD, Desai J, Fletcher JA, Morgan JA, Fletcher CDM, Kazanovicz A, et al. SU11248, a multi-targeted tyrosine kinase inhibitor, can overcome imatinib (IM) resistance caused by diverse genomic mechanisms in patients (pts) with metastatic gastrointestinal stromal tumor (GIST) [abstract]. 2004 ASCO Annual Meeting Proceedings. J Clin Oncol. 2004. 22:Abstract No 3001.21. Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005. 11:4182–4190.22. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006. 368:1329–1338.23. DeMatteo R, Owzar K, Maki R, Pisters P, Blackstein M, Antonescu C, et al. Adjuvant imatinib mesylate increases recurrence free survival (RFS) in patients with completely resected localized primary gastrointestinal stromal tumor (GIST): North American Intergroup Phase III trial ACOSOG Z9001 [abstract]. 2007 ASCO Annual Meeting. J Clin Oncol. 2007. 25:Abstract No 10079.24. Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006. 244:176–184.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastrointestinal Stromal Tumors: Case Report, Aeromedical Assessment of Therapy
- Usefulness of DOG1 Expression in the Diagnosis of Gastrointestinal Stromal Tumors
- Prognosis of Gastrointestinal Stromal Tumors Arising in the Stomach and Small Intestine: A Retrospective Study of 126 Cases from a Single Institution
- Laparoscopic Wedge Resection of GISTs Involving the Second Portion of the Duodenum
- Multiple Gastrointestinal Stromal Tumors of the Small Intestine