J Korean Ophthalmol Soc.
2008 Mar;49(3):531-538. 10.3341/jkos.2008.49.3.531.
A Case of Valganciclovir Treatment for Cytomegalovirus Retinitis
- Affiliations
-
- 1Department of Ophthalmology, Inha University School of Medicine, Incheon, Koera.
- 2Ophthalmologic Oncology Clinic, National Cancer Center, Gyeonggi, Korea. dreye@freechal.com
- KMID: 2211331
- DOI: http://doi.org/10.3341/jkos.2008.49.3.531
Abstract
-
PURPOSE: To report a case of a patient with cytomegalovirus (CMV) retinitis who was treated with oral valganciclovir.
CASE SUMMARY
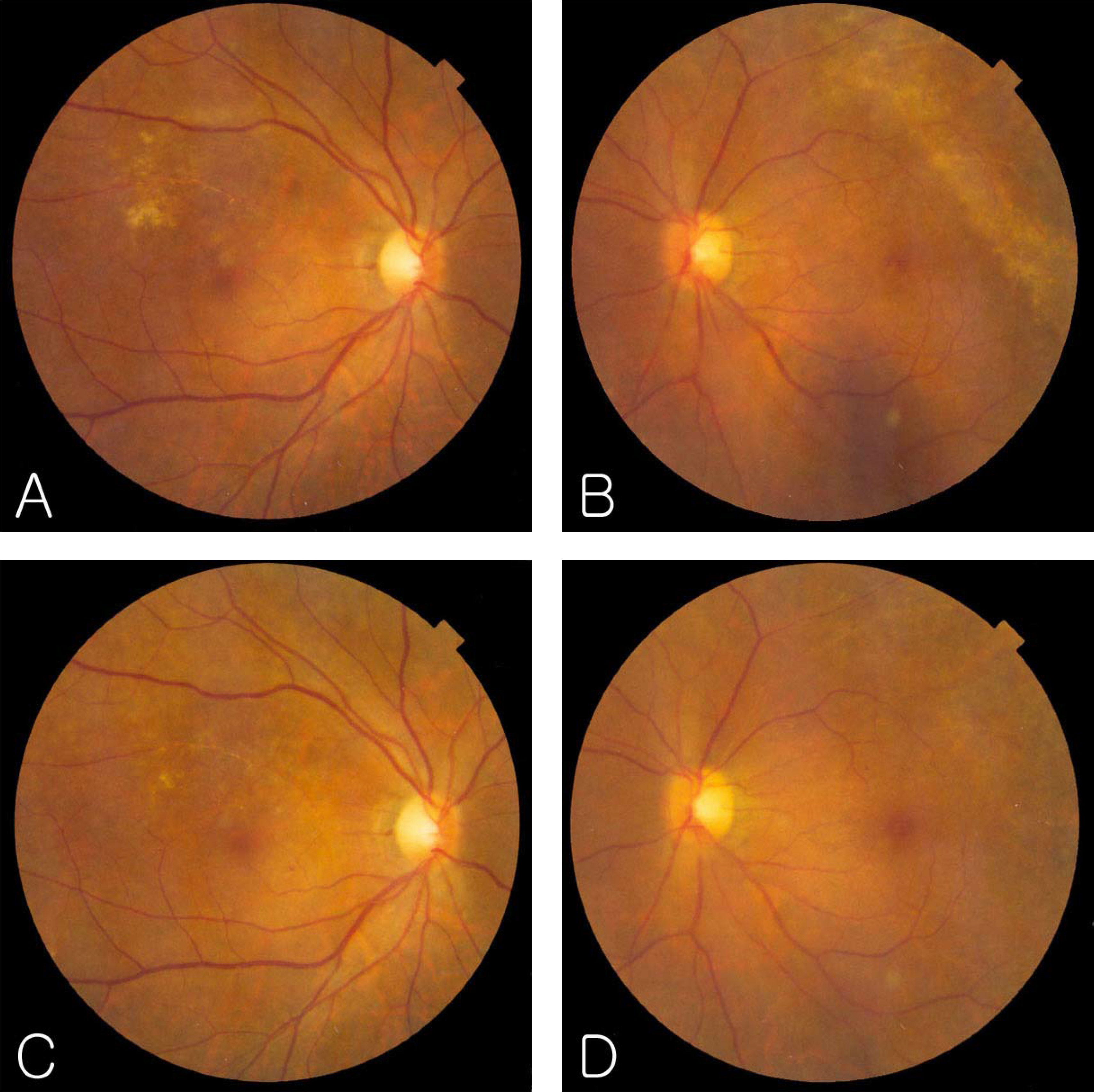
A 34-year-old man who had undergone anti-cancer chemotherapy for Non-Hodgkin lymphoma was referred to the ophthalmologic oncology clinic because of decreased vision in both eyes. Fundus examination showed white, opaque, and granular retinal lesions in both eyes, and a serologic test showed a positive response to CMV antibody IgG and a negative response to CMV antibody IgM. The patient received induction therapy with intravenous ganciclovir and maintenance therapy with oral valganciclovir 900 mg once daily. CMV retinitis reactivated 4 weeks after maintenance therapy was discontinued. At that point, the patient received induction therapy with oral valganciclovir 900 mg twice daily for 3 weeks and maintenance therapy with 900 mg once daily for 5 weeks. The retinal lesion disappeared and did not recur after oral administration of valganciclovir. The patient discontinued valganciclovir after 5 weeks of maintenance therapy, and CMV retinitis did not reactivate during 6 months of follow-up.
CONCLUSIONS
Oral valganciclovir was clinically effective in the treatment of CMV retinitis in a patient who was treated with anti-cancer chemotherapy for non-Hodgkin lymphoma.
MeSH Terms
Figure
Cited by 1 articles
-
Cinical Manifestations and Prognosis of Cytomegalovirus Retinitis
Young Kyo Kwun, Ju Byung Chae, Don Il Ham
J Korean Ophthalmol Soc. 2010;51(2):203-209. doi: 10.3341/jkos.2010.51.2.203.
Reference
-
References
1. Egbert PR, Pollard Rb, Gallagher JG, et al. Cytomegalovirus retinitis in immunosuppressed hosts. II. Ocular manifestations. Ann Intern Med. 1980; 93:664–70.2. Pescoviz MD, Rabkin J, Merion RM, et al. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. Antimicrob Agents Chemother. 2000; 44:2811–5.
Article3. Limaye AP, Corey L, Koelle DM, et al. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet. 2000; 356:645–9.
Article4. Markham A, Faulds D. Ganciclovir. An update of its therapeutic use in cytomegalovirus infection. Drugs. 1994; 48:455–84.5. Pescovitz MD, Pruett TI, Gonwa T, et al. Oral ganciclovir dosing in transplant recipients and dialysis patients based on renal function. Transplantation. 1998; 66:1104–7.6. Bartlett JG. The Johns Hopkins Hospital 1997 guide to medical care of patients with HIV infection. 7th ed.Baltimore: Williams & Wilkins;1997. p. 107–9.7. Einsele H, Reusser P, Bornhauser M, et al. Oral valganciclovir leads to higher exposure to ganciclovir than intravenous ganciclovir in patients following allogeneic stem cell transplantation. Blood. 2006; 107:3002–8.
Article8. Faulds D, Heel RC. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs. 1994; 48:455–84.9. Hamzeh FM, Literman PS. Intranuclear accumulation of sub-genomic noninfectious human cytomegalovirus DNA in infected cells in the presence of ganciclovir. Antimicrob Agents Chemother. 1991; 35:1818–23.
Article10. Jung D, Dorr A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J Clin Pharmacol. 1999; 39:800–4.
Article11. Brown F, Banken L, Saywell K, Arum I. Pharmoacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-serppositive volunteers. Clin Pharmacokinet. 1999; 37:167–76.12. Martin DF, Sierra-Madero J, Walmsley S, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002; 346:1119–26.
Article13. Kim IT, Lim JH, Seo HD. Ganciclovir treatment for cytomegalovirus retinitis in renal transplant recipients. J Korean Ophthalmol Soc. 1997; 38:242–50.14. Holland GN, Sidikaro Y, Kreiger AE, et al. Treatment of cytomegalovirus retinopathy with ganciclovir. Ophthalmology. 1987; 94:815–23.
Article15. Jabs DA, Newman C, de Bustros S, et al. Treatment of cytomegalovirus retinitis with ganciclovir. Ophthalmology. 1987; 94:824–30.
Article16. Kim YH, Kim SK. Cytomegalovirus retinitis in a child with acute lymphoblastic leukemia. J Korean Ophthalmol Soc. 2006; 47:1009–15.17. Polland RB, Egbert PR, Gallagher JG, Merigan TC. Cyto-megalovirus retinitis in immunosuppressed hosts. I. Natural history and effects of treatment with adenine arabinoside. Ann Intern Med. 1980; 93:655–64.18. Meredith TA, Aaberg TM, Resser FH. Rhegmatogenous retinal detachment complicating cytomegalovirus retinitis. Am J Ophthalmol. 1979; 87:793–6.
Article19. Erice A, Jordan MC, Chace BA, et al. Ganciclovir treatment of cytomegalovirus disease in transplant recipients and other immunocompromised hosts. JAMA. 1987; 12:3082–7.
Article20. Harbison MA, DeGirolami PC, Jenkins RL, et al. Ganciclovir therapy of severe cytomegalovirus infections in solid organ transplant recipients. Transplantation. 1988; 46:82–8.21. Banmal CR, Levin AV, Read SE, et al. Cytomegalovirus retinitis in immunosupressed children. Am J Ophthalmol. 1999; 127:550–8.22. Park MY, Ohn YH, Park SH. Three cases of cytomegalovirus retinitis in the immunosupressed kidney transplant patients. J Korean Ophthalmol Soc. 1993; 34:918–23.23. Yi WM, Kim MH, Yoo JS, Huh W. CMV papillitis in renal transplant recipient. J Korean Ophthalmol Soc. 1998; 39:2768–71.24. Cho CW, Park YM, Seo MS. Effect of ganciclovir on cytomegalovirus retinitis of a renal transplant patient without maintenance therapy. J Korean Ophthalmol Soc. 1997; 38:637–42.25. Lalezari J, lindely J, Walmsley S, et al. A safety study of oral valganciclovir maintenance treatment of cytomegalovirus retinitis. J Acquir Immune Defic Syndr. 2002; 30:392–400.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytomegalovirus Retinitis in a Hematopoietic Stem Cell Transplant Recipient During Maribavir Pre-emptive Therapy
- Therapeutic Effect of Ganciclovir on Cytomegalovirus Retinitis
- Ocular Ischemic Syndrome as the Initial Presenting Feature of Cytomegalovirus Retinitis
- 4 Cases of Reactivated Cytomegalovirus Retinitis in Immunocompromised Patients
- Cytomegalovirus Retinopathy in Aequired Immunodeficieney Syndrome