J Korean Soc Radiol.
2013 Aug;69(2):105-112. 10.3348/jksr.2013.69.2.105.
Measurement of the Aortic Diameter in the Asymptomatic Korean Population: Assessment with Multidetector CT
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine and Institute of Radiation Medicine, SNUMRC (Seoul National University Medical Research Center), Clinical Research Institute, Seoul National University Hospital, Seoul, Korea. whal.lee
- KMID: 2208813
- DOI: http://doi.org/10.3348/jksr.2013.69.2.105
Abstract
- PURPOSE
To determine normal reference values for aortic diameters in asymptomatic Korean adults.
MATERIALS AND METHODS
Three hundred adults without signs or symptoms of cardiovascular diseases were enrolled in this study. Aortic diameters were measured at nine predetermined levels on CT images. Aortic diameter measurements were adjusted for body surface area. Analysis of data was performed with regard to age, sex, weight, height and hypertension.
RESULTS
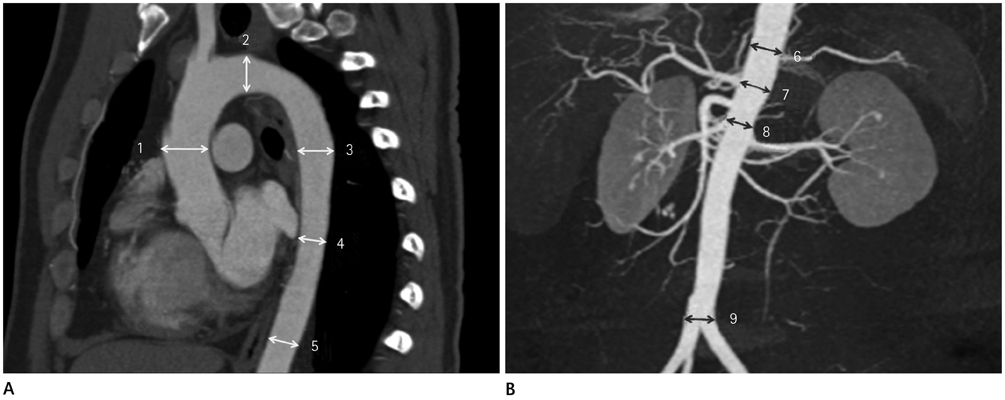
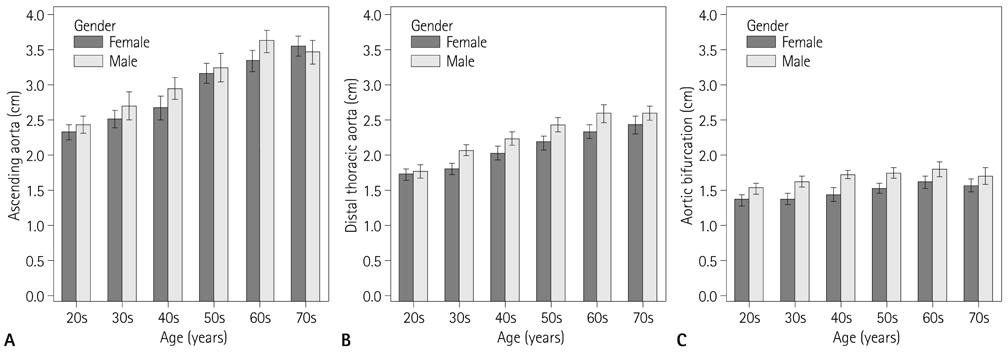
Aortic diameters were 2.99 +/- 0.57 cm at the ascending aorta, 2.54 +/- 0.35 cm at the transverse aortic arch, 2.36 +/- 0.35 cm at the proximal descending thoracic aorta (DTA), 2.23 +/- 0.37 cm at the mid DTA, 2.17 +/- 0.38 cm at the distal DTA, 2.16 +/- 0.37 cm at the thoracoabdominal junction, 2.10 +/- 0.35 cm at the level of the celiac axis, 1.94 +/- 0.36 cm at the suprarenal aorta, 1.58 +/- 0.24 cm at the aortic bifurcation. Men had slightly larger diameters than women (p < 0.05). All diameters increased with age and hypertension, with statistical significance (p < 0.01). And all aortic diameters increased with height (p < 0.05) except at the level of the aortic arch (p = 0.056), and increased with weight (p < 0.05) except at the level of the suprarenal aorta (p = 0.067).
CONCLUSION
Male sex, higher weight and height, age and hypertension are associated with larger aortic diameters in asymptomatic Korean adults.
MeSH Terms
Figure
Reference
-
1. Trerotola SO. Can helical CT replace aortography in thoracic trauma. Radiology. 1995; 197:13–15.2. Sommer T, Fehske W, Holzknecht N, Smekal AV, Keller E, Lutterbey G, et al. Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging. Radiology. 1996; 199:347–352.3. Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA. 1998; 280:1926–1929.4. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010; 121:e266–e369.5. Lu TL, Huber CH, Rizzo E, Dehmeshki J, von Segesser LK, Qanadli SD. Ascending aorta measurements as assessed by ECG-gated multi-detector computed tomography: a pilot study to establish normative values for transcatheter therapies. Eur Radiol. 2009; 19:664–669.6. Guthaner DF, Wexler L, Harell G. CT demonstration of cardiac structures. AJR Am J Roentgenol. 1979; 133:75–81.7. Aronberg DJ, Glazer HS, Madsen K, Sagel SS. Normal thoracic aortic diameters by computed tomography. J Comput Assist Tomogr. 1984; 8:247–250.8. Horejs D, Gilbert PM, Burstein S, Vogelzang RL. Normal aortoiliac diameters by CT. J Comput Assist Tomogr. 1988; 12:602–603.9. Dixon AK, Lawrence JP, Mitchell JR. Age-related changes in the abdominal aorta shown by computed tomography. Clin Radiol. 1984; 35:33–37.10. Hager A, Kaemmerer H, Rapp-Bernhardt U, Blücher S, Rapp K, Bernhardt TM, et al. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg. 2002; 123:1060–1066.11. Wolak A, Gransar H, Thomson LE, Friedman JD, Hachamovitch R, Gutstein A, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging. 2008; 1:200–209.12. Mao SS, Ahmadi N, Shah B, Beckmann D, Chen A, Ngo L, et al. Normal thoracic aorta diameter on cardiac computed tomography in healthy asymptomatic adults: impact of age and gender. Acad Radiol. 2008; 15:827–834.13. Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A, et al. Assessment of the thoracic aorta by multidetector computed tomography: age- and sex-specific reference values in adults without evident cardiovascular disease. J Cardiovasc Comput Tomogr. 2008; 2:298–308.14. Euathrongchit J, Deesuwan P, Kuanprasert S, Woragitpoopol S. Normal thoracic aortic diameter in Thai people by multidetector computed tomography. J Med Assoc Thai. 2009; 92:236–242.15. Vasan RS, Larson MG, Levy D. Determinants of echocardiographic aortic root size. The Framingham Heart Study. Circulation. 1995; 91:734–774.16. Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989; 64:507–512.17. Cohen GI, White M, Sochowski RA, Klein AL, Bridge PD, Stewart WJ, et al. Reference values for normal adult transesophageal echocardiographic measurements. J Am Soc Echocardiogr. 1995; 8:221–230.18. Reed CM, Richey PA, Pulliam DA, Somes GW, Alpert BS. Aortic dimensions in tall men and women. Am J Cardiol. 1993; 71:608–610.19. Kim M, Roman MJ, Cavallini MC, Schwartz JE, Pickering TG, Devereux RB. Effect of hypertension on aortic root size and prevalence of aortic regurgitation. Hypertension. 1996; 28:47–52.20. Jakrapanichakul D, Chirakarnjanakorn S. Comparison of aortic diameter in normal subjects and patients with systemic hypertension. J Med Assoc Thai. 2011; 94:Suppl 1. S51–S56.21. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006; 27:2588–2605.22. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010; 121:505–511.23. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010; 55:1318–1327.24. O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007; 50:1–13.25. ILSI Risk Science Institute Working Group. Physiological Parameter Values for PBPK Models. Washington D.C: ILSI;1994. p. 40–66.26. Moon DH, Lee HK, Song HC, Lee J, Bom HS, Sohn HK, et al. Change of cerebral blood flow distribution and vascular reserve according to age in Koreans measured by Tc-99m HMPAO brain SPECT. Korean J Nucl Med. 1999; 33:247–261.27. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007; 62:847–855.28. Hatazawa J, Iida H, Shimosegawa E, Sato T, Murakami M, Miura Y. Regional cerebral blood flow measurement with iodine-123-IMP autoradiography: normal values, reproducibility and sensitivity to hypoperfusion. J Nucl Med. 1997; 38:1102–1108.29. Gur RE, Gur RC. Gender differences in regional cerebral blood flow. Schizophr Bull. 1990; 16:247–254.30. Esposito G, Van Horn JD, Weinberger DR, Berman KF. Gender differences in cerebral blood flow as a function of cognitive state with PET. J Nucl Med. 1996; 37:559–564.31. Horiguchi J, Kiguchi M, Fujioka C, Shen Y, Arie R, Sunasaka K, et al. Radiation dose, image quality, stenosis measurement, and CT densitometry using ECG-triggered coronary 64-MDCT angiography: a phantom study. AJR Am J Roentgenol. 2008; 190:315–320.32. van Prehn J, Vincken KL, Muhs BE, Barwegen GK, Bartels LW, Prokop M, et al. Toward endografting of the ascending aorta: insight into dynamics using dynamic cine-CTA. J Endovasc Ther. 2007; 14:551–560.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diameters of the Thoracic Aorta Measured with Multidetector Computed Tomography
- Acute Aortic Syndrome: Recent Trends in Imaging Assessment Using Computed Tomography Angiography
- Determination of Diameter and Angulation of the Normal Common Bile Duct using Multidetector Computed Tomography
- Multidetector Computed Tomography Findings of a Papillary Fibroelastoma of the Aortic Valve: A Case Report
- Aortic Arch Variants and Anomalies: Embryology, Imaging Findings, and Clinical Considerations