J Korean Assoc Oral Maxillofac Surg.
2010 Apr;36(2):87-93. 10.5125/jkaoms.2010.36.2.87.
Maxillary sinus floor elevation using autogenous skin-derived mesenchymal stem cells in miniature pigs
- Affiliations
-
- 1Department Oral and Maxillofacial Surgery, School of Medicine and Institute of Health Science, Gyeongsang National University, Jinju, Korea. parkbw@gsnu.ac.kr
- 2College of Veterinary Medicine, Gyeongsang National University, Jinju, Korea.
- 3Department of Neurosurgery, School of Medicine, Gyeongsang National University, Jinju, Korea.
- 4Department of Pathology, School of Medicine, Gyeongsang National University, Jinju, Korea.
- KMID: 2189976
- DOI: http://doi.org/10.5125/jkaoms.2010.36.2.87
Abstract
- INTRODUCTION
In our previous studies, we isolated porcine skin-derived mesenchymal stem cells (pSDMSCs) from the ears of adult miniature pigs and evaluated the pluripotency of these pSDMSCs based on expressions of transcription factors, such as Oct-4, Sox-2, and Nanog. Moreover, the characteristic of mesenchymal stem cells was revealed by the expression of various mesenchymal stem cell markers, including CD29, CD44, CD90, and vimentin. The aim of this study was to evaluate in vivo osteogenesis after maxillary sinus lift procedures with autogenous pSDMSCs and scaffold.
MATERIALS AND METHODS
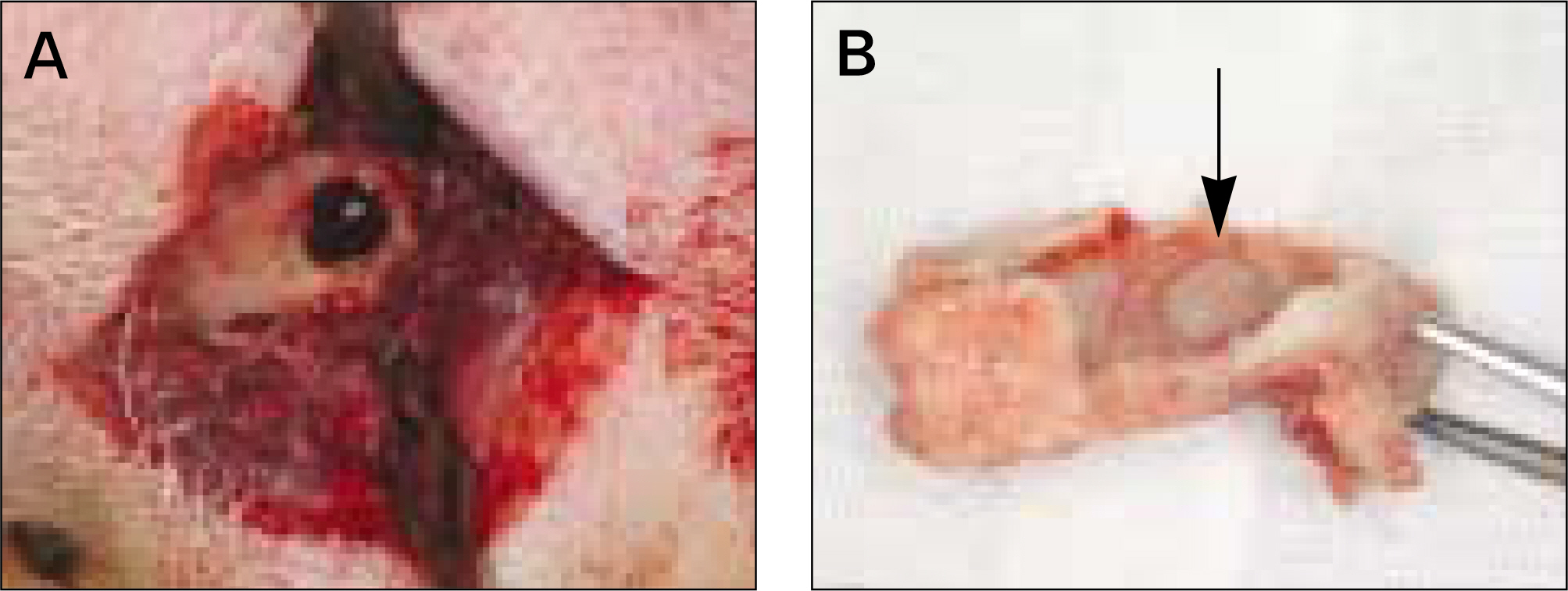
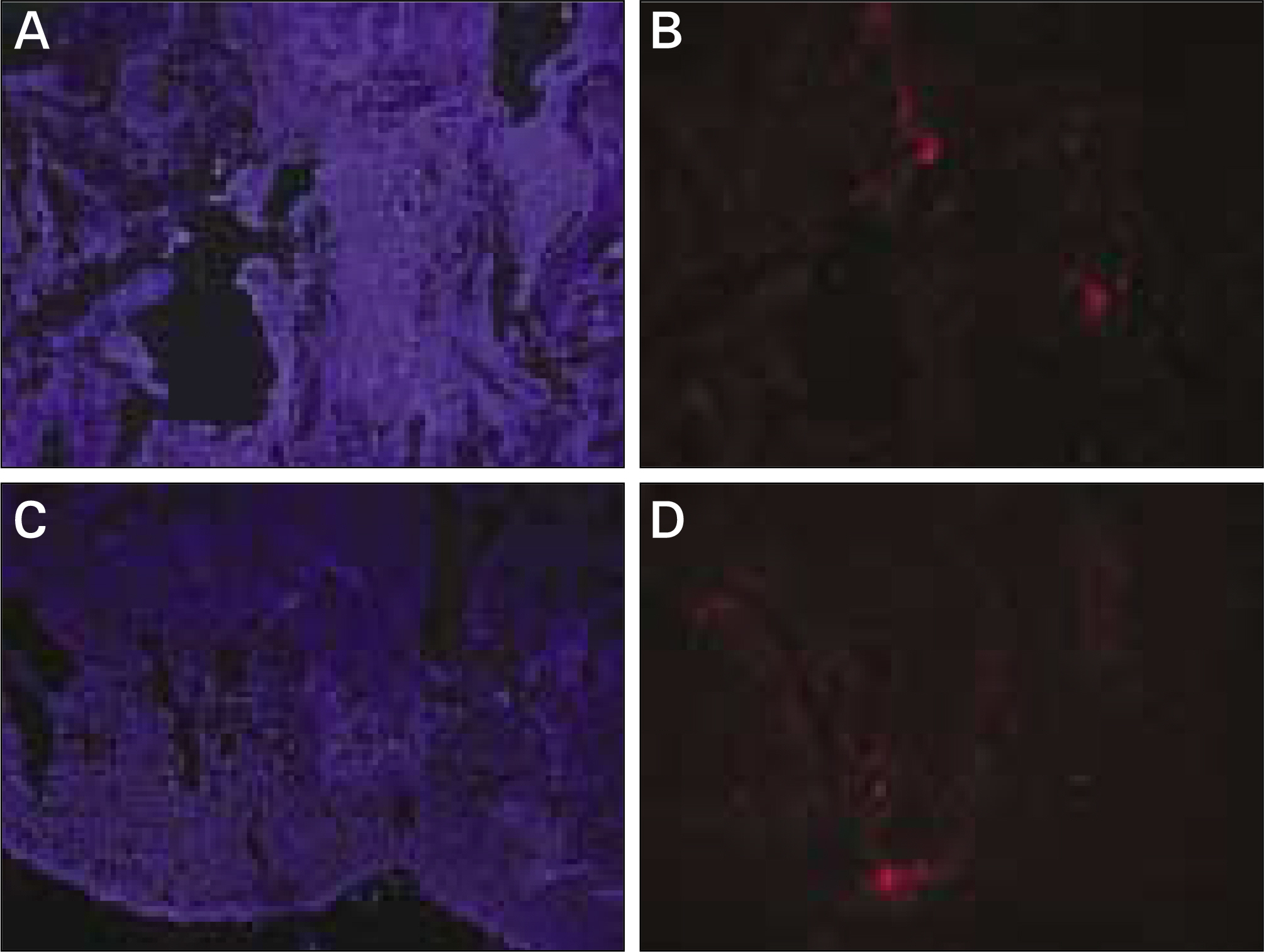
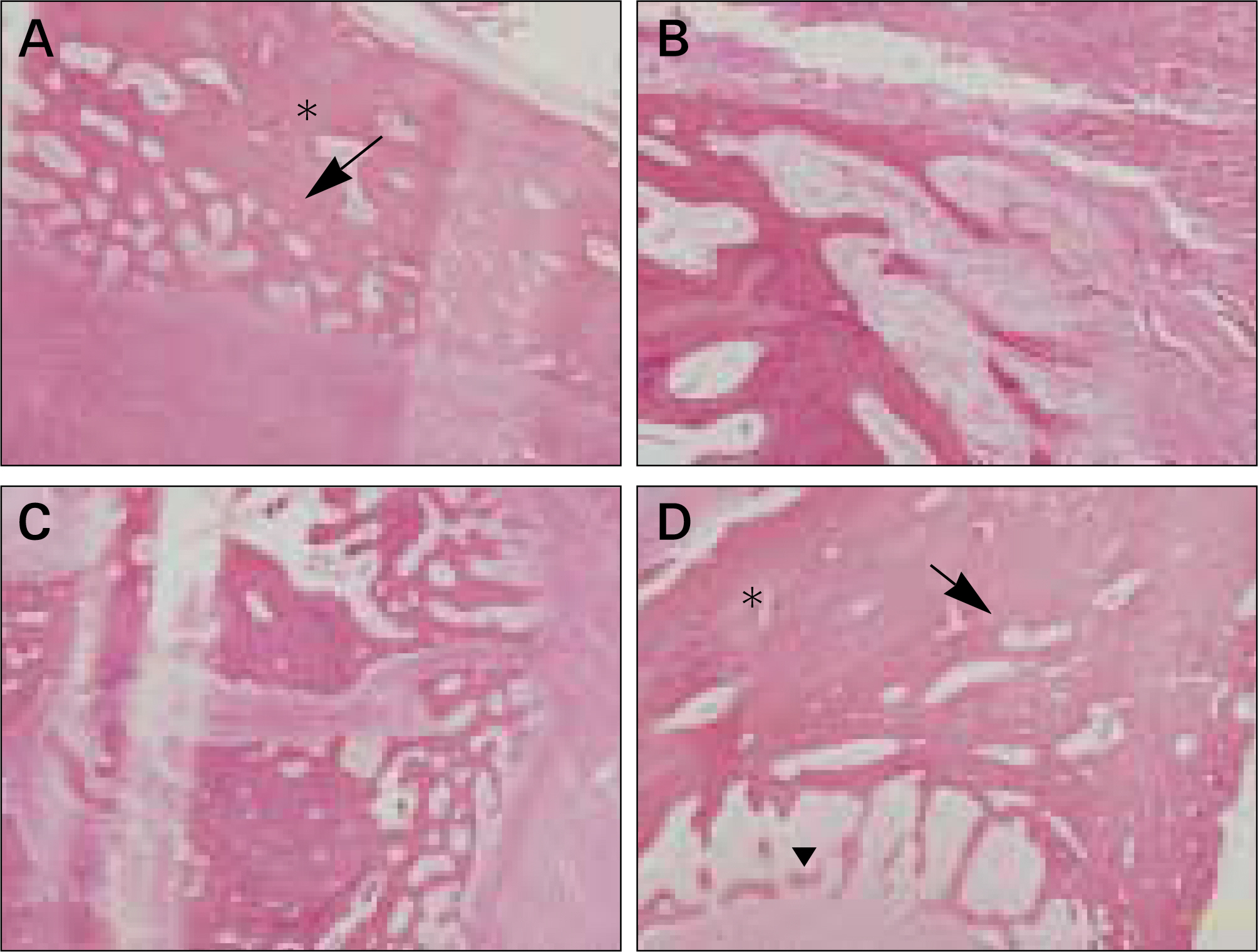
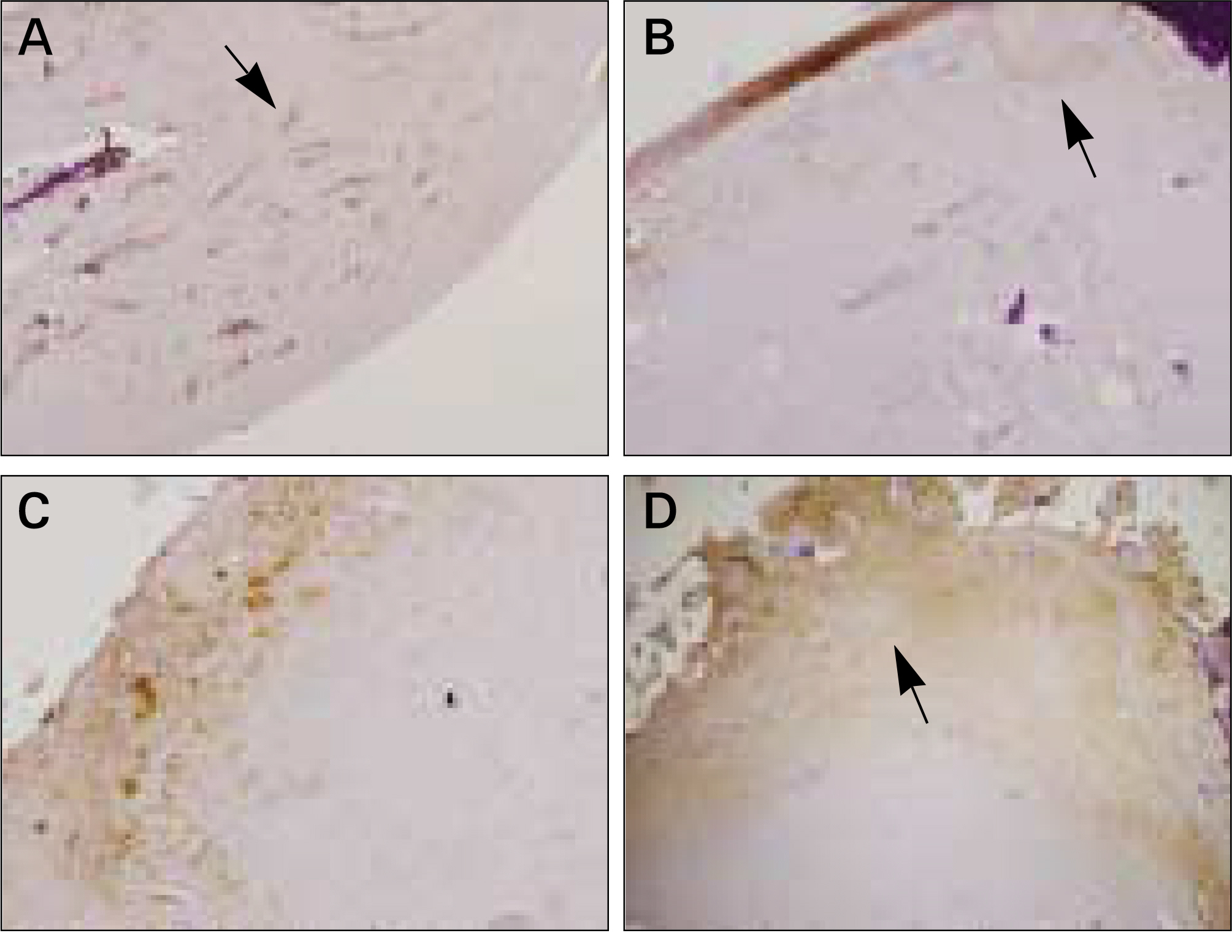
The autogenous pSDMSCs were isolated from the 4 miniature pigs, and cultured to 3rd passage with same methods of our previous studies. After cell membranes were labeled using a PKH26, 1x10(7) cells/100 microliter of autogenous pSDMSCs were grafted into the maxillary sinus with a demineralized bone matrix (DBM) and fibrin glue scaffold. In the contralateral control side, only a scaffold was grafted, without SDMSCs. After two animals each were euthanized at 2 and 4 weeks after grafting, the in vivo osteogenesis was evaluated with histolomorphometric and osteocalcin immunohistochemical studies.
RESULTS
In vivo PKH26 expression was detected in all specimens at 2 and 4 weeks after grafting. Trabecular bone formation and osteocalcin expression were more pronounced around the grafted materials in the autogenous pSDMSCs-grafted group compared to the control group. Newly generated bone was observed growing from the periphery to the center of the grafted material.
CONCLUSION
The results of the present study suggest that autogenous skin-derived mesenchymal stem cells grafting with a DBM and fibrin glue scaffold can be a predictable method in the maxillary sinus floor elevation technique for implant surgery.
Keyword
MeSH Terms
-
Adult
Animals
Bone Matrix
Cell Membrane
Ear
Fibrin Tissue Adhesive
Floors and Floorcoverings
Humans
Maxillary Sinus
Mesenchymal Stromal Cells
Organic Chemicals
Osteocalcin
Osteogenesis
Swine
Tissue Engineering
Transcription Factors
Transplants
Vimentin
Fibrin Tissue Adhesive
Organic Chemicals
Osteocalcin
Transcription Factors
Vimentin
Figure
Cited by 1 articles
-
Regenerative medicine for the reconstruction of hard tissue defects in oral and maxillofacial surgery
Young-Kyun Kim
J Korean Assoc Oral Maxillofac Surg. 2012;38(2):69-70. doi: 10.5125/jkaoms.2012.38.2.69.
Reference
-
References
1. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980; 38:613–6.2. Tatum H Jr. Maxillary and sinus Implant reconstructions. Dent Clin North Am. 1986; 30:207–29.3. Schmelzeisen R, Schimming R, Sittinger M. Making bone: implant insertion into tissue-engineered bone for maxillary sinus floor augmentation-a preliminary report. J Craniomaxillofac Surg. 2003; 31:34–94.
Article4. Wheeler SL, Holmes RE, Calhoun CJ. Six-year clinical and histologic study of sinus-lift grafts. Int J Oral Maxillofac Implants. 1996; 11:26–34.5. Vacanti JP. Beyond transplantation. Third annual Samuel Jason Mixter lecture. Arch Surg. 1988; 123:545–9.6. Chen F, Feng X, Wu W, Ouyang H, Gao Z, Cheng X, et al. Segmental bone tissue engineering by seeding osteoblast precursor cells into titanium mesh-coral composite scaffolds. Int J Oral Maxillofac Surg. 2007; 36:822–7.
Article7. Yamada Y, Boo JS, Ozawa R, Nagasaka T, Okazaki Y, Hata K, et al. Bone regeneration following injection of mesenchymal stem cells and fibrin glue with a biodegradable scaffold. J Craniomaxillofac Surg. 2003; 31:27–33.
Article8. Park BW, Hah YS, Kim DR, Kim JR, Byun JH. Osteogenic phenotypes and mineralization of cultured human periosteal-derived cells. Arch Oral Biol. 2007; 52:983–9.
Article9. Schimming R, Schmelzeisen R. Tissue-engineered bone for maxillary sinus augmentation. J Oral Maxillofac Surg. 2004; 62:724–9.
Article10. Fuerst G, Tangl S, Gruber R, Gahleitner A, Sanroman F, Watzek G. Bone formation following sinus grafting with autogenous bone-derived cells and bovine bone mineral in minipigs: preliminary findings. Clin Oral Implants Res. 2004; 15:733–40.
Article11. Shi C, Zhu Y, Su Y, Cheng T. Stem cells and their applications in skin-cell therapy. Trends Biotechnol. 2006; 24:48–52.
Article12. Riekstina U, Muceniece R, Cakstina I, Muiznieks I, Ancans J. Characterization of human skin-derived mesenchymal stem cell proliferation rate in different growth conditions. Cytotechnology. 2008; 58:153–62.
Article13. Blanpain C, Fuchs E. Epidermal stem cells of skin. Annu Rev Cell Dev Biol. 2006; 22:339–73.14. Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006; 116:249–60.
Article15. Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001; 3:778–84.
Article16. Dyce PW, Zhu H, Craig J, Li J. Stem cells with multilineage potential derived from porcine skin. Biochem Biophys Res Commun. 2004; 316:651–8.
Article17. Fernandes KJ, Toma JG, Miller FD. Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos Trans R Soc Lond B Biol Sci. 2008; 363:185–98.
Article18. Rho GJ, Kumar BM, Balasubramanian SS. Porcine mesenchymal stem cells-current technological status and future perspective. Front Biosci. 2009; 14:3942–61.19. Choi MJ, Byun JH, Kang EJ, Rho GJ, Kim UK, Kim JR, et al. Isolation of porcine multipotential skin-derived precursor cells and its multilineage differentiation. J Korean Assoc Oral Maxillofac Surg. 2008; 34:588–93.20. Byun JH, Choi MJ, Choi YJ, Shim KM, Kim UK, Kim JR, et al. Mandibular bone regeneration using autogenous skin-derived precursor cells with mixed demineralized bone and fibrin glue scaffold in miniature pigs. J Korean Assoc Maillofac Plast Reconstr Surg. 2009; 31:198–206.21. Kang EJ, Byun JH, Choi YJ, Maeng GH, Lee SL, Kang DH, et al. In vitro and in vivo osteogenesis of porcine skin-derived mesenchymal stem cell-like cells with a demineralized bone and fibrin scaffold. Tissue Eng Part A 201016:. 815–27.22. Kajahn J, Gorjup E, Tiede S, von Briesen H, Paus R, Kruse C, et al. Skin-derived human adult stem cells surprisingly share many features with human pancreatic stem cells. Eur J Cell Biol. 2008; 87:39–46.
Article23. Perka C, Schultz O, Spitzer RS, Lindenhayn K, Burmester GR, Sittinger M. Segmental bone repair by tissue-engineered periosteal cell transplants with bioresorbable fleece and fibrin scaffolds in rabbits. Biomaterials. 2000; 21:1145–53.
Article24. Schwarz N, Redl H, Schlag G, Schiesser A, Lintner F, Dinges HP, et al. The influence of fibrin sealant on demineralized bone matrix-dependent osteoinduction. A quantitative and qualitative study in rats. Clin Orthop Relat Res. 1989; 238:282–7.25. Pieri F, Lucarelli E, Corinaldesi G, Fini M, Aldini NN, Giardino R, et al. Effect of mesenchymal stem cells and platelet-rich plasma on the healing of standardized bone defects in the alveolar ridge: a comparative histomorphometric study in minipigs. J Oral Maxillofac Surg. 2009; 67:265–72.
Article26. Klongnoi B, Rupprecht S, Kessler P, Thorwarth M, Wiltfang J, Schlegel KA. Influence of platelet-rich plasma on a bioglass and autogenous bone in sinus augmentation. An explorative study. Clin Oral Implants Res. 2006; 17:312–20.
Article27. Fu ¨rst G, Gruber R, Tangl S, Zechner W, Haas R, Mailath G, et al. Sinus grafting with autogenous platelet-rich plasma and bovine hydroxyapatite. A histomorphometric study in minipigs. Clin Oral Implants Res. 2003; 14:500–8.28. Byun JH, Park BW, Kim JR, Lee JH. Expression of vascular endothelial growth factor and its receptors after mandibular distraction osteogenesis. Int J Oral Maxillofac Surg. 2007; 36:338–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of undifferentiated mesenchymal stem cells on sinus bone grafting in rabbit
- Osteogenic potential of adult stem cells from human maxillary sinus membrane by Simvastatin in vitro: preliminary report
- The use of Autologous Venous Blood for Maxillary Sinus Floor Augmentation in Conjunction with the Sinus Membrane Elevation: An Experimental Study
- Maxillary sinus bone graft using particulated ramal autobone and bovine bone
- An 8-year follow-up of autotransplantation into artificial socket with sinus elevation and autogenous bone grafting