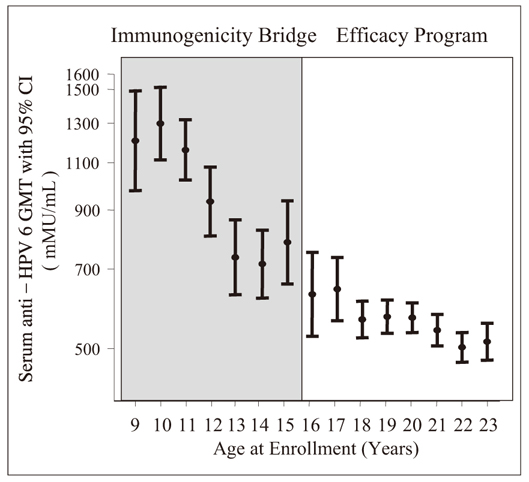
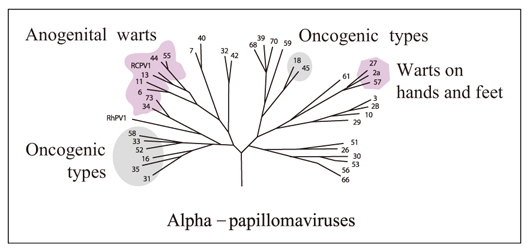
J Korean Med Assoc.
2008 Feb;51(2):144-157. 10.5124/jkma.2008.51.2.144.
Human Papillomavirus Vaccine
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University College of Medicine, Korea. kaykim@ewha.ac.kr
- KMID: 2185732
- DOI: http://doi.org/10.5124/jkma.2008.51.2.144
Abstract
- Vaccination has had a major effect on protection of humans against disease. The human papillomavirus (HPV) vaccine is the most recent breakthrough in the vaccine development, which provides an exceptional opportunity for cancer prevention through vaccination. The prophylactic quadrivalent vaccine using L1 virus-like particles (VLPs) of HPV 6, 11, 16, and 18 is now available in Korea, and the bivalent vaccine containing VLPs of HPV16 and HPV18 is available in other countries. Results from the phase IIb and III trials show that these two HPV vaccines are safe and well-tolerated. They offer HPV-naive women a very high level of efficacy against persistent infection and cervical intra-epithelial lesions associated with the HPV types included in the vaccine. The quadrivalent vaccine has also been shown to protect against vulvar and vaginal lesions and genital warts caused by types 6 and 11. Recent studies on both vaccines indicate there is a high level of cross-protection against infections associated with other related oncogenic HPV types, which are responsible for a further 10% of cervical cancers globally. The Korean Society of Pediatrics has developed guidelines for the use of the prophylactic HPV vaccine for the prevention of cervical intraepithelial neoplasia and cervical cancer. They address the use of prophylactic HPV vaccines, including who should be vaccinated and at what age, as well as a summary of policy and implementation issues. With the introduction of the vaccines, general issues have to be raised such as duration of protection after vaccination, data on different immunization schedules, data on infants and young children, cross-protection, impact on cervical cancer screening, vaccination of males, potential replacement infection and vaccine compatibility. This review provides an up-to-date summary of recent studies and available information concerning HPV vaccination.
Keyword
MeSH Terms
Figure
Reference
-
1. Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006. 116:1167–1173.
Article2. Pollack AE, Balkin M, Edouard L, Cutts F, Broutet N. WHO/UNFPA Working Group on Sexual and Reproductive Health and HPV Vaccines. Ensuring access to HPV vaccines through integrated services: a reproductive health perspective. Bull World Health Organ. 2007. 85:57–63.
Article3. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices(ACIP). MMWR Recomm Rep. 2007. 56(RR-2):1–24.4. American Academy of Pediatrics Committee on Infectious Diseases. Recommended immunization schedules for children and adolescents-United States, 2007. Pediatrics. 2007. 119:207–208.5. Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, Goldie SJ, Harper DM, Kinney W, Moscicki AB, Noller KL, Wheeler CM, Ades T, Andrews KS, Doroshenk MK, Kahn KG, Schmidt C, Shafey O, Smith RA, Partridge EE, Garcia F. Gynecologic Cancer Advisory Group. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007. 57:7–28.
Article6. Kim KH, Kim JH, Park SE, Shin SH, Oh SH, Lee HJ, Jo DS, Choi EH, Hur JK, Hong YJ. The Committee on Infectious Diseases, The Korean Pediatric Society. Human Papillomavirus Vaccine. Korean J Pediatr. 2007. 50:811–819.7. Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998. 338:423–428.
Article8. Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, Darragh TM, Brescia R, Kanowitz S, Miller SB, Stone J, Hanson E, Palefsky J. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998. 132:277–284.
Article9. Ho GY, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P, Basu J, Tachezy R, Lewis R, Romney S. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995. 87:1365–1371.
Article10. Schiffman M, Kjaer SK. Natural history of anogenital human papillomavirus infection and neoplasia. J Natl Cancer Inst Monogr. 2003. 31:14–19.11. de Villiers EM, Fauquet C, Broker TR, Bernard HU, Zur HH. Classification of papillomaviruses. Virology. 2004. 324:17–27.
Article12. Bonnez W, Reichman RC. Mandell GL, Bennet JE, Dolin R, editors. Papillomaviruses. Principles and practice of Infectious Disease. 2005. 6th ed. Elsevior Inc.;1841–1855.
Article13. Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003. 348:518–527.
Article14. Clifford G, Franceschi S, Diaz M, Muñoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006. 24:S3. 26–34.
Article15. Duensing S, Munger K. Mechanisms of genomic instability in human cancer: insights from studies with human papillomavirus oncoproteins. Int J Cancer. 2004. 109:157–162.
Article16. Stanley M. Immune responses to human papillomavirus. Vaccine. 2006. 24:S16–S22.
Article17. Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000. 181:1911–1919.
Article18. Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003. 157:218–226.
Article19. Manhart LE, Holmes KK, Koutsky LA, Wood TR, Kenney DL, Feng Q, Kiviat NB. Human papillomavirus infection among sexually active young women in the United States: implications for developing a vaccination strategy. Sex Transm Dis. 2006. 33:502–508.
Article20. Watts DH, Koutsky LA, Holmes KK, Goldman D, Kuypers J, Kiviat NB, Galloway DA. Low risk of perinatal transmission of human papillomavirus: results from a prospective cohort study. Am J Obstet Gynecol. 1998. 178:365–373.
Article21. Moscicki AB, Schiffman M, Kjaer S, Villa LL. Updating the natural history of HPV and anogenital cancer. Vaccine. 2006. 24:S42–S51.22. Castellsague X, Munoz N. Cofactors in human papillomavirus carcinogenesis-role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003. 31:20–28.23. Ministry of Health and Welfare. 2002 Annual Report of the Korea Central Cancer Registry. (Published in 2003).24. Korea National Statistical Office. Annual Report on the Cause of Death Statistics. (published in 2005).25. Shin HR, Lee DH, Herrero R, Smith JS, Vaccarella S, Hong SH, Jung KY, Kim HH, Park UD, Cha HS, Park S, Touze A, Muñoz N, Snijders PJ, Meijer CJ, Coursaget P, Franceschi S. Prevalence of human papillomavirus infection in women in Busan, South Korea. Int J Cancer. 2003. 103:413–421.
Article26. Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, Kong HJ, Rha SH, Jung SI, Kim JI, Jung KY, van Doorn LJ, Quint W. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004. 190:468–476.
Article27. Shin HR, Seoh K, Song KJ, Park EC, Choi GS, Lee HY, Oh JK, Kim BK, Kim JY. A report of investigation of disease burden associated with human papillomavirus infection in Korea. Korean Center for Disease Control and Prevention;(published in 2007).28. Stanley M, Lowy DR, Frazer I. Prophylactic vaccines: underlying mechanisms. Vaccine. 2006. 24:S106–S113.29. Kahn JA. Vaccination as a prevention strategy for human papillomavirus-related diseases. J Adolesc Health. 2005. 37:S6. S10–S16.
Article30. Noad R, Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003. 11:438–444.
Article31. Kim YT. Prophylactic vaccine for cervical carcinoma. J Korean Med Assoc. 2007. 50:151–158.
Article32. Ahn WS. Recent concepts for human papillomavirus vaccine. J Korean Med Assoc. 2007. 50:778–784.33. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, de Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccineagainst infection with human papillomavirus types 16 and 18 in young women: aninterim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007. 369:2161–2170.
Article34. Ault KA. Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007. 369:1861–1868.
Article35. Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GW, Ferris DG, Steben M, Bryan J, Taddeo FJ, Railkar R, Esser MT, Sings HL, Nelson M, Boslego J, Sattler C, Barr E, Koutsky LA. Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007. 356:1928–1943.
Article36. Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Høye J, Steinwall M, Riis-Johannessen G, Andersson-Ellstrom A, Elfgren K, KroghG , Lehtinen M, Malm C, Tamms GM, Giacoletti K, Lupinacci L, Railkar R, Taddeo FJ, Bryan J, Esser MT, Sings HL, Saah AJ, Barr E. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006. 95:1459–1466.
Article37. Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Høye J, Steinwall M, Riis-Johannessen G, Andersson-Ellstrom A, Elfgren K, von Krogh G, Lehtinen M, Paavonen J, Tamms GM, Giacoletti K, Lupinacci L, Esser MT, Vuocolo SC, Saah AJ, Barr E. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007. 25:4931–4939.
Article38. Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bilvalent L1virus-like particle vaccine against human papillomavirus types 16 and 18: follow up from a randomised control trial. Lancet. 2006. 367:1247–1255.
Article39. Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003. 10:108–115.
Article40. Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, Brown M, Smith J, Chirmule N, Barr E, Jansen KU, Esser MT. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005. 12:959–969.
Article41. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, Roteli-Martins CM, Teixeira J, Blatter MM, Korn AP, Quint W, Dubin G. GlaxoSmithKline HPV Vaccine Study Group. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004. 364:1757–1765.
Article42. Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, Puchalski D, Giacoletti KE, Sings HL, Lukac S, Alvarez FB, Barr E. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007. 26:201–209.
Article43. Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsagué X, Rusche SA, Lukac S, Bryan JT, Cavanaugh PF Jr, Reisinger KS. Protocol 016 Study Group. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006. 118:2135–2145.
Article44. Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, Brown DR, Ferenczy A, Harper DM, Koutsky LA, Kurman RJ, Lehtinen M, Malm C, Olsson SE, Ronnett BM, Skjeldestad FE, Steinwall M, Stoler MH, Wheeler CM, Taddeo FJ, Yu J, Lupinacci L, Railkar R, Marchese R, Esser MT, Bryan J, Jansen KU, Sings HL, TammsGM , Saah AJ, Barr E. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine. 2006. 24:5571–5583.
Article45. Kang S, Kim KH, Kim YT, Kim YT, Kim JH, Song YS, Shin SH, Ryu HS, Han JW, Kang JH, Park SY. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, placebo-controlled trial in 176 Korean subjects. Int J Gynecol Cancer. 2007.
Article46. Munoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006. 24:S3. 1–10.
Article47. Brown D. FUTURE Study Group. HPV Type 6/11/16/18 Vaccine: First analysis of cross-protection against persistent infection, cervical intraepithelial neoplasia (CIN), and adenocarcinoma in situ (AIS) caused by oncogenic HPV types in addition to 16/18. 2007. 09. Chicago: ICAAC;September 2007.48. Product approval information-licensing action [package insert]. Gardasil (quadrivalent human papillomavirus types 6, 11, 16, 18). Food and Drug Administration. Whitehouse Station, NJ: Merck & Co.;Available at http://www.fda.gov/cber/label/HPVmer060806LB.pdf.49. Preparing for the introduction of HPV vaccines, Policy and programme guidance for countries. http://www.fda.gov/cber/products/hpvmer060806.htm.50. Soldan K, Dillner J. Comparable strategies needed to evaluate human papillomavirus vaccine efficiency across Europe. Euro Surveill. 2006. 11:E061123.3.
Article51. Kudjawu Y, Lévy-Bruhl D, Celentano LP, O'Flanagan D, Salmaso S, Lopalco P, Mullins N, Bacci S. VENICE working group. The current status of HPV and rotavirus vaccines in national immunisation schedules in the EU-preliminary results of a VENICE survey. Euro Surveill. 2007. 12:E070426.1.52. Howell-Jones R. Human papillomavirus vaccination: the United Kingdom's recommendation and update on European licensure and efficacy data. Euro Surveill. 2007. 12:E071115.4.
Article