J Gastric Cancer.
2014 Dec;14(4):271-274. 10.5230/jgc.2014.14.4.271.
A Rare Presentation of Metastasis of Prostate Adenocarcinoma to the Stomach and Rectum
- Affiliations
-
- 1Division of Hematology/Oncology, The Brooklyn Hospital Center, Brooklyn, NY, USA. a.minsoe77@gmail.com
- 2Department of Pathology, The Brooklyn Hospital Center, Brooklyn, NY, USA.
- 3Division of Gastroenterology, The Brooklyn Hospital Center, Brooklyn, NY, USA.
- KMID: 2183652
- DOI: http://doi.org/10.5230/jgc.2014.14.4.271
Abstract
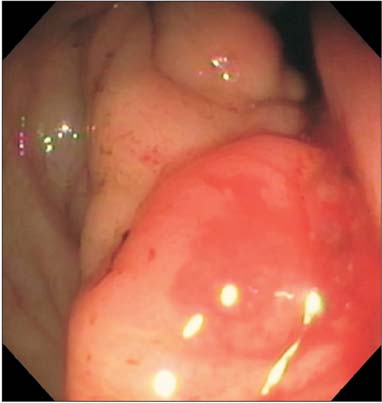
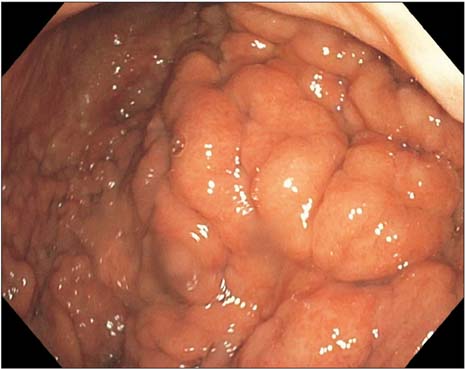
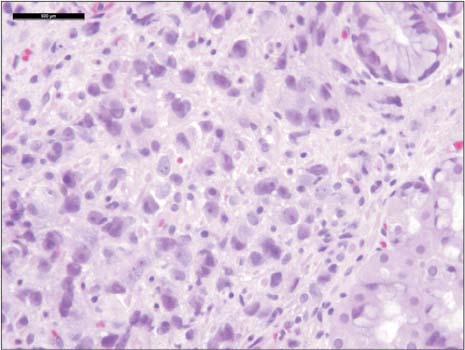
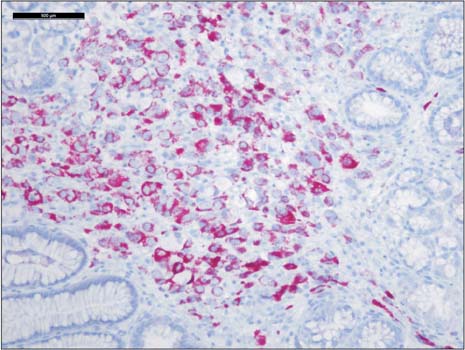
- Prostate cancer is the second most common cause of cancer death in men in the United States. The most common sites of metastasis include the bone, lymph nodes, lung, liver, pleura, and adrenal glands, whereas metastatic prostate cancer involving the gastrointestinal tract has been rarely reported. A 64-year-old African-American man with a history of prostate cancer presented with anemia. He reported the passing of dark colored stools but denied hematemesis or hematochezia. Colonoscopy revealed circumferential nodularity, and histology demonstrated metastatic carcinoma of the prostate. Esophagogastroduodenoscopy showed hypertrophic folds in the gastric fundus, and microscopic examination revealed tumor cells positive for prostate-specific antigen. Bone scanning and computed tomography of the abdomen and pelvis did not show metastasis. It is crucial to distinguish primary gastrointestinal cancer from metastatic lesions, especially in patients with a history of cancer at another site, for appropriate management.
MeSH Terms
-
Abdomen
Adenocarcinoma*
Adrenal Glands
Anemia
Colonoscopy
Endoscopy, Digestive System
Gastric Fundus
Gastrointestinal Hemorrhage
Gastrointestinal Neoplasms
Gastrointestinal Tract
Hematemesis
Humans
Liver
Lung
Lymph Nodes
Male
Middle Aged
Neoplasm Metastasis*
Pelvis
Pleura
Prostate*
Prostate-Specific Antigen
Prostatic Neoplasms
Rectum*
Stomach*
United States
Prostate-Specific Antigen
Figure
Reference
-
1. National Cancer Institute (NCI). SEER cancer status review 1975-2011 [Internet]. USA: NCI;cited 2014 Jun 15. Available from: http://seer.cancer.gov/csr/1975_2011/results_merged/sect_23_prostate.pdf.2. Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000; 31:578–583.
Article3. Christoph F, Grünbaum M, Wolkers F, Müller M, Miller K. Prostate cancer metastatic to the stomach. Urology. 2004; 63:778–779.
Article4. Ben-Izhak O, Lichtig C. Signet-ring cell carcinoma of the prostate mimicking primary gastric carcinoma. J Clin Pathol. 1992; 45:452–454.
Article5. Holderman WH, Jacques JM, Blackstone MO, Brasitus TA. Prostate cancer metastatic to the stomach. Clinical aspects and endoscopic diagnosis. J Clin Gastroenterol. 1992; 14:251–254.
Article6. Green LK. Hematogenous metastases to the stomach. A review of 67 cases. Cancer. 1990; 65:1596–1600.
Article7. Nakamura T, Mohri H, Shimazaki M, Ito Y, Ohnishi T, Nishino Y, et al. Esophageal metastasis from prostate cancer: diagnostic use of reverse transcriptase-polymerase chain reaction for prostate-specific antigen. J Gastroenterol. 1997; 32:236–240.
Article8. Gore RM, Sparberg M. Metastatic carcinoma of the prostate to the esophagus. Am J Gastroenterol. 1982; 77:358–359.9. Eaves R, Lambert J, Rees J, King RW. Achalasia secondary to carcinoma of prostate. Dig Dis Sci. 1983; 28:278–284.
Article10. Malhi-Chowla N, Wolfsen HC, Menke D, Woodward TA. Prostate cancer metastasizing to the small bowel. J Clin Gastroenterol. 2001; 32:439–440.
Article11. Onitilo AA, Engel JM, Resnick JM. Prostate carcinoma metastatic to the stomach: report of two cases and review of the literature. Clin Med Res. 2010; 8:18–21.
Article12. Kolonel LN. Cancer patterns of four ethnic groups in Hawaii. J Natl Cancer Inst. 1980; 65:1127–1139.13. Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000; 31:578–583.
Article14. Menuck LS, Amberg JR. Metastatic disease involving the stomach. Am J Dig Dis. 1975; 20:903–913.
Article15. Oda I, Kondo H, Yamao T, Saito D, Ono H, Gotoda T, et al. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy. 2001; 33:507–510.
Article16. Mintz ER, Smith GG. Autopsy findings in 100 cases of prostate carcinoma. N Eng J Med. 1934; 211:479–487.17. Jiang Z, Wu CL, Woda BA, Dresser K, Xu J, Fanger GR, et al. P504S/alpha-methylacyl-CoA racemase: a useful marker for diagnosis of small foci of prostatic carcinoma on needle biopsy. Am J Surg Pathol. 2002; 26:1169–1174.18. Beach R, Gown AM, De Peralta-Venturina MN, Folpe AL, Yaziji H, Salles PG, et al. P504S immunohistochemical detection in 405 prostatic specimens including 376 18-gauge needle biopsies. Am J Surg Pathol. 2002; 26:1588–1596.
Article