J Korean Diabetes Assoc.
2006 Jul;30(4):237-245. 10.4093/jkda.2006.30.4.237.
Rho-kinase and Insulin Signaling
- Affiliations
-
- 1Division of Endocrinology, Diabetes and Metabolism, Beth Israel Deaconess Medical Center and Harvard Medical School, USA.
- KMID: 2177593
- DOI: http://doi.org/10.4093/jkda.2006.30.4.237
Abstract
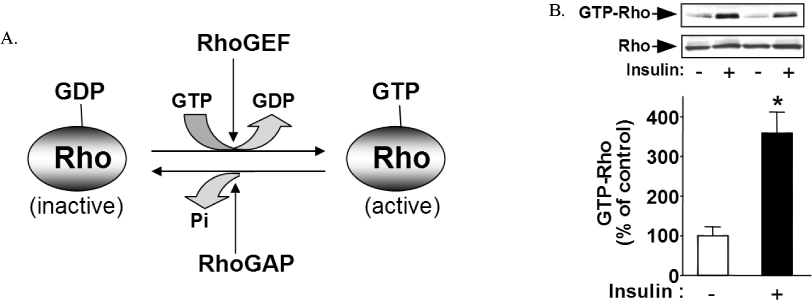
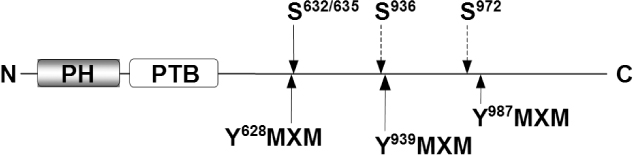
- Insulin's ability to acutely stimulate glucose uptake and metabolism in peripheral tissues is essential for normal glucose homeostasis. Resistance to this insulin effect is a major pathogenic feature of type 2 diabetes and obesity. Although many of the proximal steps in insulin signaling have been identified, the molecular mechanisms underlying insulin resistance under various metabolic states are still unclear. Recent study suggests that Rho-kinase is an important mediator of insulin signaling and glucose homeostasis. Specifically, Rho-kinase directly controls insulin receptor substrate-1, which plays an important role in regulating insulin action. Inhibition of Rho-kinase function results in a decreased insulin response, leading to insulin resistance. Thus, Rho-kinase is identified as a novel regulator of insulin action and glucose homeostasis, and a potential target for new diabetes drugs.
MeSH Terms
Figure
Reference
-
1. DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes. Diabetes Rev. 1997. 5:177–269.2. Kahn BB. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998. 92:593–596.3. White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997. 40:Suppl 2. S2–S17.4. Goldstein BJ, Ahmad F, Ding W, Li PM, Zhang WR. Regulation of the insulin signalling pathway by cellular protein- tyrosine phosphatases. Mol Cell Biochem. 1998. 182:91–99.5. Cheatham B, Kahn CR. Insulin action and the insulin signalling network. Endocrin Rev. 1995. 16:117–142.6. Frevert EU, Kahn BB. Differential effects of constitutively active phosphatidylinositol 3-kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Mol Cell Biol. 1997. 17:190–198.7. Holman GD, Kasuga M. From receptor to transporter: insulin signalling to glucose transport. Diabetologia. 1997. 40:991–1003.8. Farah S, Agazie Y, Ohan N, Ngsee JK, Liu XJ. A rho-associated protein kinase, ROKalpha, binds insulin receptor substrate-1 and modulates insulin signaling. J Biol Chem. 1998. 273:4740–4746.9. Begum N, Sandu OA, Ito M, Lohmann SM, Smolenski A. Active Rho kinase (ROK-alpha) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem. 2002. 277:6214–6222.10. Symons M, Settleman J. Rho family GTPases: more than simple switches. Trends Cell Biol. 2000. 10:415–419.11. Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001. 81:153–208.12. Gong MC, Fujihara H, Somlyo AV, Somlyo AP. Translocation of rhoA associated with Ca2+ sensitization of smooth muscle. J Biol Chem. 1997. 272:10704–10709.13. Karnam P, Standaert ML, Galloway L, Farese RV. Activation and translocation of Rho (and ADP ribosylation factor) by insulin in rat adipocytes. Apparent involvement of phosphatidylinositol 3-kinase. J Biol Chem. 1997. 272:6136–6140.14. Standaert M, Bandyopadhyay G, Galloway L, Ono Y, Mukai H, Farese R. Comparative effects of GTPgammaS and insulin on the activation of Rho, phosphatidylinositol 3-kinase, and protein kinase N in rat adipocytes. Relationship to glucose transport. J Biol Chem. 1998. 273:7470–7477.15. Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metabolism. 2005. 2:119–129.16. Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. Embo J. 1996. 15:2208–2216.17. Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995. 270:29051–29054.18. Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996. 392:189–193.19. Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. Embo J. 1996. 15:1885–1893.20. Hashimoto R, Nakamura Y, Kosako H, Amano M, Kaibuchi K, Inagaki M, Takeda M. Distribution of Rho-kinase in the bovine brain. Biochem Biophys Res Commun. 1999. 263:575–579.21. Komagome R, Kimura K, Saito M. Postnatal changes in Rho and Rho-related proteins in the mouse brain. Jpn J Vet Res. 2000. 47:127–133.22. Sin WC, Chen XQ, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol. 1998. 18:6325–6339.23. Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000. 11:1709–1725.24. Chevrier V, Piel M, Collomb N, Saoudi Y, Frank R, Paintrand M, Narumiya S, Bornens M, Job D. The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J Cell Biol. 2002. 157:807–817.25. Amano M, Chihara K, Nakamura N, Kaneko T, Matsuura Y, Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates rho-kinase activity. J Biol Chem. 1999. 274:32418–32424.26. Feng J, Ito M, Kureishi Y, Ichikawa K, Amano M, Isaka N, Okawa K, Iwamatsu A, Kaibuchi K, Hartshorne DJ, Nakano T. Rho-associated kinase of chicken gizzard smooth muscle. J Biol Chem. 1999. 274:3744–3752.27. Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001. 3:346–352.28. Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001. 22:32–39.29. Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho- kinase. Science. 1997. 275:1308–1311.30. Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996. 16:5313–5327.31. Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996. 273:245–248.32. Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999. 274:37385–37390.33. Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997. 389:990–994.34. Sordella R, Classon M, Hu KQ, Matheson SF, Brouns MR, Fine B, Zhang L, Takami H, Yamada Y, Settleman J. Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev Cell. 2002. 2:553–565.35. Sordella R, Jiang W, Chen GC, Curto M, Settleman J. Modulation of Rho GTPase Signaling Regulates a Switch between Adipogenesis and Myogenesis. Cell. 2003. 113:147–158.36. Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997. 11:2295–2322.37. Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999. 68:459–486.38. Omata W, Shibata H, Li L, Takata K, Kojima I. Actin filaments play a critical role in insulin-induced exocytotic recruitment but not in endocytosis of GLUT4 in isolated rat adipocytes. Biochem J. 2000. 346(Pt 2):321–328.39. Patki V, Buxton J, Chawla A, Lifshitz L, Fogarty K, Carrington W, Tuft R, Corvera S. Insulin action on GLUT4 traffic visualized in single 3T3-l1 adipocytes by using ultra-fast microscopy. Mol Biol Cell. 2001. 12:129–141.40. Tsakiridis T, Tong P, Matthews B, Tsiani E, Bilan PJ, Klip A, Downey GP. Role of the actin cytoskeleton in insulin action. Microsc Res Tech. 1999. 47:79–92.41. Chiang SH, Baumann CA, Kanzaki M, Thurmond DC, Watson RT, Neudauer CL, Macara IG, Pessin JE, Saltiel AR. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature. 2001. 410:944–948.42. Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000. 261:44–51.43. Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med. 2002. 80:629–638.44. Shibata H, Mukai H, Inagaki Y, Homma Y, Kimura K, Kaibuchi K, Narumiya S, Ono Y. Characterization of the interaction between RhoA and the amino-terminal region of PKN. FEBS Lett. 1996. 385:221–224.45. Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998. 394:491–494.46. Reid T, Furuyashiki T, Ishizaki T, Watanabe G, Watanabe N, Fujisawa K, Morii N, Madaule P, Narumiya S. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J Biol Chem. 1996. 271:13556–13560.47. Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka A, Narumiya S. Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase Rho. Science. 1996. 271:645–648.48. Ohan N, Agazie Y, Cummings C, Booth R, Bayaa M, Liu XJ. RHO-associated protein kinase alpha potentiates insulin-induced MAP kinase activation in Xenopus oocytes. J Cell Sci. 1999. 112(Pt 13):2177–2184.49. Zick Y. Insulin resistance: a phosphorylation-based uncoupling of insulin signaling. Trends Cell Biol. 2001. 11:437–441.50. Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000. 275:9047–9054.51. De Fea K, Roth RA. Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry. 1997. 36:12939–12947.52. Greene MW, Morrice N, Garofalo RS, Roth RA. Modulation of human insulin receptor substrate-1 tyrosine phosphorylation by protein kinase Cdelta. Biochem J. 2004. 378:105–116.53. Moeschel K, Beck A, Weigert C, Lammers R, Kalbacher H, Voelter W, Schleicher ED, Haring HU, Lehmann R. Protein kinase C-zeta-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem. 2004. 279:25157–25163.54. Liu YF, Paz K, Herschkovitz A, Alt A, Tennenbaum T, Sampson SR, Ohba M, Kuroki T, LeRoith D, Zick Y. Insulin stimulates PKCzeta -mediated phosphorylation of insulin receptor substrate-1 (IRS-1). A self -attenuated mechanism to negatively regulate the function of IRS proteins. J Biol Chem. 2001. 276:14459–14465.55. Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci U S A. 2001. 98:4640–4645.56. De Fea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. J Biol Chem. 1997. 272:31400–31406.57. Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5'-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001. 276:46912–46916.58. Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004. 431:200–205.59. Qiao LY, Zhande R, Jetton TL, Zhou G, Sun XJ. In vivo phosphorylation of insulin receptor substrate 1 at serine 789 by a novel serine kinase in insulin -resistant rodents. J Biol Chem. 2002. 277:26530–26539.60. White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002. 283:E413–E422.61. Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K, Takamatsu I, Sugano N, Hayashi K, Saruta T. Rho-kinase as a molecular target for insulin resistance and hypertension. Faseb J. 2006. 20:169–171.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Mechanism of Insulin Resistance in Obesity and Type 2 Diabetes
- Role of Rho-Kinase Activity in Angiotensin II-Induced Contraction of Corpus Cavernosum Smooth Muscle in the Rabbit
- Insulin-Like Growth Factor 1 Actions in Developing Brain and the Interaction with Wnt Pathway
- Expression patterns of Rho-associated protein kinase signaling pathway-related genes in mouse submandibular glands
- Ras Mitogen-activated Protein Kinase Signaling and Kinase Suppressor of Ras as Therapeutic Targets for Hepatocellular Carcinoma