J Gynecol Oncol.
2012 Jan;23(1):22-27. 10.3802/jgo.2012.23.1.22.
Sequential chemotherapy and radiotherapy as sandwich therapy for the treatment of high risk endometrial cancer
- Affiliations
-
- 1Gynecologic Oncology Associates, Newport Beach, CA, USA. bram@gynoncology.com
- 2The Women's Cancer Research Foundation, Newport Beach, CA, USA.
- KMID: 2177510
- DOI: http://doi.org/10.3802/jgo.2012.23.1.22
Abstract
OBJECTIVE
The purpose of this retrospective study was to assess the tolerability and efficacy of sequential chemotherapy and radiotherapy for the treatment of high risk endometrial cancer.
METHODS
We conducted a retrospective study of previously untreated high risk endometrial cancer patients who received sequential chemotherapy and radiotherapy in accordance with the sandwich approach from June 2008 until June 2011. High risk endometrial cancer patients underwent complete surgical staging followed by adjuvant therapy encompassing sequential chemotherapy, radiation therapy and consolidation chemotherapy.
RESULTS
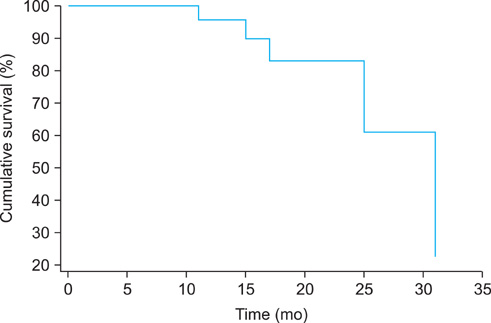
The study analysis comprised 32 endometrial cancer patients. All subjects were treated with carboplatin and paclitaxel chemotherapy; currently, 186 cycles have been administered and 94% of patients have completed the planned number of cycles. Grade 3 neutropenia developed in 1 (3.1%) patient; there was no incidence of grade 4 neutropenia. Moreover, we observed grade 3 anemia in four (12.5%) patients and grade 4 anemia in one (3.1%) patient. One (3.1%) patient developed grade 3 thrombocytopenia; grade 4 thrombocytopenia was not observed. Five patients exhibited progressive disease, three of whom have since expired; mean progression free survival and follow-up were 17.4 months and 18.9 months, respectively.
CONCLUSION
The preliminary results from our study suggest that the sandwich approach to treating high risk endometrial cancer patients is feasible. Hematologic toxicity was well tolerated and non-hematologic toxicity was mild and easily managed. Further study of this novel regimen in a larger patient population with extended follow-up is necessary.
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.2. Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006. 24:36–44.3. Smith RS, Kapp DS, Chen Q, Teng NN. Treatment of high-risk uterine cancer with whole abdominopelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2000. 48:767–778.4. Onda T, Yoshikawa H, Mizutani K, Mishima M, Yokota H, Nagano H, et al. Treatment of node-positive endometrial cancer with complete node dissection, chemotherapy and radiation therapy. Br J Cancer. 1997. 75:1836–1841.5. Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J Clin Oncol. 2004. 22:3902–3908.6. Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley HD, Malfetano JH, et al. Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a gynecologic oncology group study. Gynecol Oncol. 2005. 97:755–763.7. Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004. 22:2159–2166.8. Aoki Y, Kase H, Watanabe M, Sato T, Kurata H, Tanaka K. Stage III endometrial cancer: analysis of prognostic factors and failure patterns after adjuvant chemotherapy. Gynecol Oncol. 2001. 83:1–5.9. Secord AA, Havrilesky LJ, O'Malley DM, Bae-Jump V, Fleming ND, Broadwater G, et al. A multicenter evaluation of sequential multimodality therapy and clinical outcome for the treatment of advanced endometrial cancer. Gynecol Oncol. 2009. 114:442–447.10. Geller MA, Ivy J, Dusenbery KE, Ghebre R, Isaksson Vogel R, Argenta PA. A single institution experience using sequential multi-modality adjuvant chemotherapy and radiation in the sandwich method for high risk endometrial carcinoma. Gynecol Oncol. 2010. 118:19–23.11. Fields AL, Einstein MH, Novetsky AP, Gebb J, Goldberg GL. Pilot phase II trial of radiation "sandwiched" between combination paclitaxel/platinum chemotherapy in patients with uterine papillary serous carcinoma (UPSC). Gynecol Oncol. 2008. 108:201–206.12. Lupe K, Kwon J, D'Souza D, Gawlik C, Stitt L, Whiston F, et al. Adjuvant paclitaxel and carboplatin chemotherapy with involved field radiation in advanced endometrial cancer: a sequential approach. Int J Radiat Oncol Biol Phys. 2007. 67:110–116.13. Alvarez Secord A, Havrilesky LJ, Bae-Jump V, Chin J, Calingaert B, Bland A, et al. The role of multi-modality adjuvant chemotherapy and radiation in women with advanced stage endometrial cancer. Gynecol Oncol. 2007. 107:285–291.14. Patsavas K, Woessner J, Gielda B, Rotmensch J, Yordan E, Bitterman P, et al. Optimal surgical debulking in uterine papillary serous carcinoma affects survival. Gynecol Oncol. 2011. 121:581–585.15. National Institute of Health. NIH common terminology criteria for adverse events (CTCAE) v3.0. 2003. Washington: National Institute of Health.16. Geller MA, Ivy JJ, Ghebre R, Downs LS Jr, Judson PL, Carson LF, et al. A phase II trial of carboplatin and docetaxel followed by radiotherapy given in a sandwich method for stage III, IV, and recurrent endometrial cancer. Gynecol Oncol. 2011. 121:112–117.17. O'Hanlan KA, Levine PA, Harbatkin D, Feiner C, Goldberg GL, Jones JG, et al. Virulence of papillary endometrial carcinoma. Gynecol Oncol. 1990. 37:112–119.18. Nout RA, Smit VT, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomized trial. Lancet. 2010. 375:816–823.19. Diavolitsis V, Boyle J, Singh DK, Small W Jr. The role of adjuvant radiation in endometrial cancer. Oncology (Williston Park). 2009. 23:342–349.20. Dickler A, Puthawala MY, Thropay JP, Bhatnagar A, Schreiber G. Prospective multi-center trial utilizing electronic brachytherapy for the treatment of endometrial cancer. Radiat Oncol. 2010. 5:67.21. McCloskey SA, Tchabo NE, Malhotra HK, Odunsi K, Rodabaugh K, Singhal P, et al. Adjuvant vaginal brachytherapy alone for high risk localized endometrial cancer as defined by the three major randomized trials of adjuvant pelvic radiation. Gynecol Oncol. 2010. 116:404–407.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Trend of the Postoperative Adjuvant Therapy in Endometrial Cancer
- A multi-institutional analysis of sequential versus ‘sandwich’ adjuvant chemotherapy and radiotherapy for stage IIIC endometrial carcinoma
- Adjuvant therapy in cervical cancer patients with high risk factors
- Adjuvant sequential chemo and radiotherapy improves the oncological outcome in high risk endometrial cancer
- Present status and future direction of clinical trials in advanced endometrial carcinoma