J Korean Acad Conserv Dent.
2010 Sep;35(5):321-334. 10.5395/JKACD.2010.35.5.321.
The effect of solvent evaporation of dentin adhesive on bonding efficacy
- Affiliations
-
- 1Division of Dentistry, Department of Conservative Dentistry, Graduate of Kyung Hee University, Seoul, Korea. choikkyu@khu.ac.kr
- KMID: 2176430
- DOI: http://doi.org/10.5395/JKACD.2010.35.5.321
Abstract
OBJECTIVES
The purpose of this study is to evaluate bonding efficacy by means of measuring the effect of remained solvent on Degree of conversion(DC) and microTBS and FE-SEM examination.
MATERIALS AND METHODS
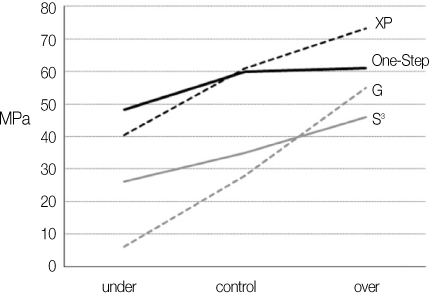
Two 2-step total etching adhesives and two single-step self etching adhesives were used in this study. First, volume weight loss of 4 dentin adhesives were measured using weighting machine in process of time in normal conditions and calculate degree of evaporation (DE). Reaction/reference intensity ratio were measured using micro-Raman spectroscopy and calculate DC according to DE. Then 2 experimental groups were prepared according to air-drying methods (under, over) and control group was prepared to manufacturer's instruction. Total 12 groups were evaluated by means of micro tensile bond strength and FE-SEM examination.
RESULTS
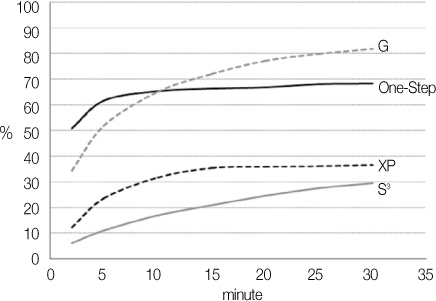
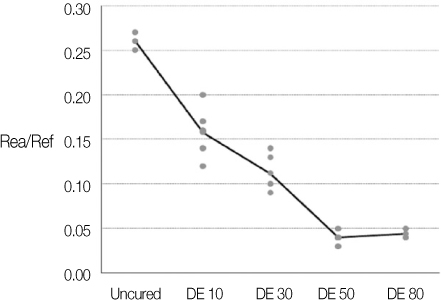
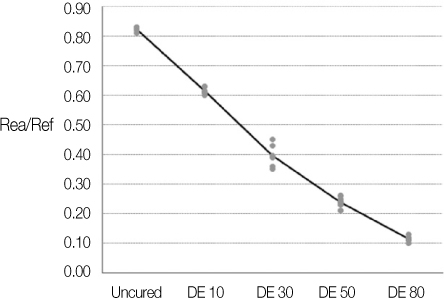
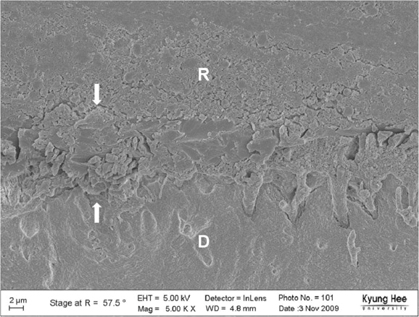
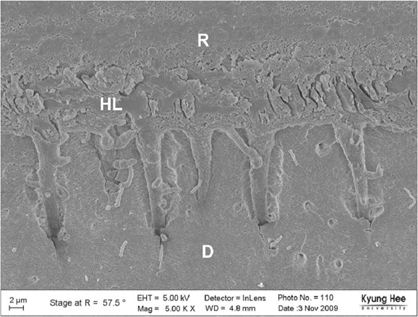
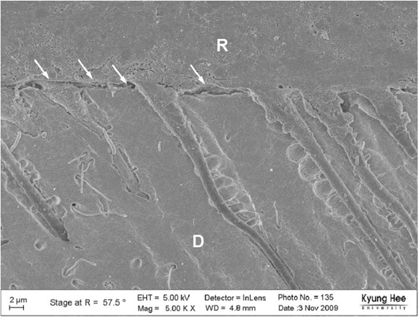
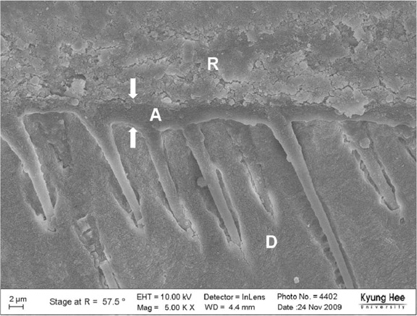
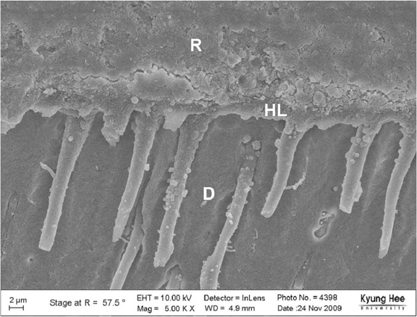
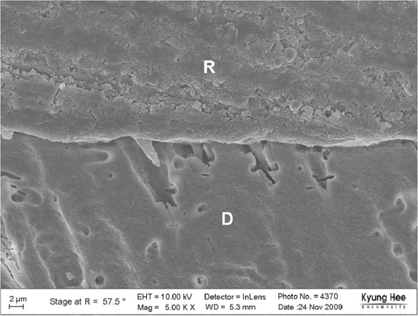
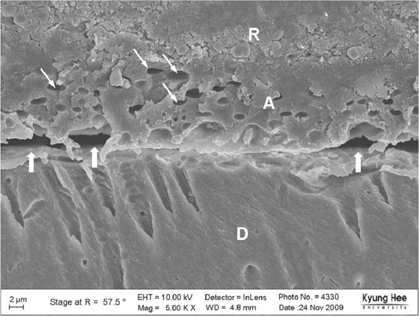
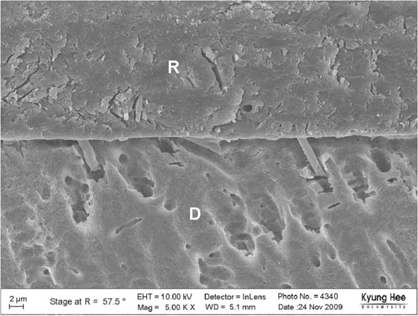
Degree of evaporation (DE) was increased as time elapsed but different features were observed according to the kind of solvents. Acetone based adhesive showed higher DE than ethanol and butanol based adhesive. Degree of conversion (DC) was increased according to DE except for S3 bond. In microTBS evaluation, bond strength was increased by additional air-drying. Large gaps and droplets were observed in acetone based adhesives by FE-SEM pictures.
CONCLUSIONS
Additional air-drying is recommended for single-step self etching adhesive but careful consideration is required for 2-step total etching adhesive because of oxygen inhibition layer. Evaporation method is carefully chose and applied according to the solvent type.
Keyword
MeSH Terms
Figure
Reference
-
1. Kanca J. Effect of resin primer and surface wetness on resin composite bond strength to dentin. Am J Dent. 1992. 5:213–215.2. Gwinnett AJ. Moist versus dry dentin: its effect on shear bond strength. Am J Dent. 1992. 5:127–129.3. Kanca J. Improving bond strength through acid etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992. 123:35–43.
Article4. Pashley DH, Carvalho RM. Dentin permeability and dentin adhesion. J Dent. 1997. 25:355–372.5. Van Meerbeek B, Perdigao J, Lambrechts P, Vanherle G. The clinical performance of adhesives. J Dent. 1998. 26:1–20.
Article6. Reis AF, Oliveria MT, Giannini M, de Goes MF, Rueggeberg FA. The effect of organic solvents on one-bottle adhesives' bond strength to enamel and dentin. Oper Dent. 2003. 28:700–706.7. Jacobsen T, Soderholm KJ. Some effects of water on dentin bonding. Dent Mater. 1995. 11:132–136.
Article8. De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J Dent Res. 2005. 84:118–132.
Article9. Ikeda T, De Munck J, Shirai K, Hikita K, Inoue S, Sano H, Lambrechts P, Van Meerbeek B. Effect of evaporation of primer components on ultimate tensile strengths of primer-adhesive mixture. Dent Mater. 2005. 21:1051–1058.
Article10. Carvalho RM, Mendonca JS, Santiago SL, Silveira RR, Garcia FC, Tay FR, Pashley DH. Effects of HEMA/solvent combinations on bond strength to dentin. J Dent Res. 2003. 82:597–601.
Article11. Tay FR, Gwinnett JA, Wei SH. Relation between water content in acetone/alcohol-based primer and interfacial ultrastructure. J Dent. 1998. 26:147–156.
Article12. Graham Solomons TW, Fryhle CB. Organic chemistry. 2004. 8th edition. John Wiley & Sons, Inc..13. Chappell RP, Cobb CM, Spencer P, Eick JD. Dentinal tubule anastomosis: a potential factor in adhesive bonding? J Prosthet Dent. 1994. 72:183–188.
Article14. Jacobsen T, Finger WJ, Kanehira M. Air-drying time of self-etching adhesives vs bonding efficacy. J Adhes Dent. 2006. 8:387–392.15. Moszner N, Salz U, Zimmermann J. Chemical aspects of self-etching enamel-dentin adhesives: a systemic review. Dent Mater. 2005. 21:895–910.
Article16. Ikemura K, Tay FR, Hironaka T, Endo T, Pashley DH. Bonding mechanism and ultrastructural interfacial analysis of a single-step adhesive to dentin. Dent Mater. 2003. 19:707–715.
Article17. Furuse AY, Peutzfeldt A, Asmussen E. Effect of evaporation of solvents from one-step, self-etching adhesives. J Adhes Dent. 2008. 10:35–39.18. Hiraishi N, Nishiyama N, Ikemura K, Yau JYY, King NM, Tagami J, Pashley DH, Tay FR. Water concentration in self-etching primers affects their aggressiveness and bonding efficacy to dentin. J Dent Res. 2005. 84:653–658.
Article19. Chiba Y, Yamaguchi K, Miyazaki M, Tsubota K, Takamizawa T, Moore BK. Effect of air-drying time of single-application self-etching adhesives on dentin bond strength. Oper Dent. 2006. 31:233–239.
Article20. Loguercio AD, Loeblein F, Cherobin T, Ogliari F, Piva E, Reis A. Effect of solvent removal on adhesive properties of simplified etch-and-rinse systems and on bond strengths to dry and wet dentin. J Adhes Dent. 2009. 213–219.21. Takahashi A, Sato Y, Uno S, Pereira PN, Sano H. Effects of mechanical properties of adhesive resins on bond strength to dentin. Dent Mater. 2002. 18:263–268.
Article22. Yanagawa T, Finger WJ. Relationship between degree of polymerization of composite resin and bond strength to Gluma-treated dentin. Am J Dent. 1994. 7:157–160.23. Giannini M, Arrais CAG, Vermelho PM, Reis RS, Santos LPS, Lrite ER. Effects of the solvent evaporation technique on the degree of conversion of one-bottle adhesive systems. Oper Dent. 2008. 33:149–154.
Article24. Ikeda T, De Munck J, Shirai K, Hikita K, Inoue S, Sano H, Lambrchts P, Van Meerbeek B. Effect of air-drying and solvent evaporation on the strength of HEMA-rich versus HEMA-free one-step adhesives. Dent Mater. 2008. 24:1316–1323.
Article25. Paul SJ, Leach M, Rueggeberg FA, Pashley DH. Effect of water content on the physical properties of model dentine primer and bonding resins. J Dent. 1999. 27:209–214.
Article26. Yiu CK, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005. 26:6863–6872.
Article27. Pianelli C, Devaux J, Bebelman S, Leloup G. The micro-Raman spectroscopy, a useful tool to determine the degree of conversion of light-activated composite resins. J Biomed Mater Res. 1999. 48:675–681.
Article28. Miyazaki M, Onose H, Iida N, Kazama H. Determination of residual double bonds in resin-dentin interface by Raman spectroscopy. Dent Mater. 2003. 19:245–251.
Article29. Zou Y, Armstrong SR, Jessop JL. Apparent conversion of adhesive resin in the hybrid layer, Part I: Identification of an internal reference for Raman spectroscopy and the effects of water storage. J Biomed Mater Res A. 2008. 86(4):883–891.30. Zou Y, Jessop JL, Armstrong SR. Apparent conversion of adhesive resin in the hybrid layer, Part II: In situ studies of the resin-dentin bond. J Biomed Mater Res A. 2009. 89(2):355–362.31. Cadenaro M, Breschi L, Rueggeberg FA, Suchko M, Grodin E, Agee K, Di Lenarda R, Tay FR, Pashley DH. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent Mater. 2009. 25:621–628.
Article32. Dickens SH, Cho BH. Interpretation of bond failure through conversion and residual solvent measurements and Weibull analyses of flexural and micro-tensile bond strength of bonding agents. Dent Mater. 2005. 21:354–364.
Article33. Hashimoto M, Tay FR, Svizero NR, de Gee AJ, Feilzer AJ, Sano H, Kaga M, Pashley DH. The effort of common errors on sealing ability of total-etching adhesives. Dent Mater. 2006. 22:560–568.34. Choi KK. Adhesion and esthetic restoration. 2009. 1st edition. Myungmun publishing Co..35. Pashley DH, Carvalho RM, Tay FR, Agree KA, Lee KW. Solvation of dried dentin matrix by water and other polar solvents. Am J Dent. 2002. 15:97–102.36. Van Landuyt KL, De Munck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, et al. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005. 84:183–188.
Article37. Van Landuyt KL, Snauwaert J, De Munck J, Coutinho E, Poitevin A, Yoshida Y, et al. Origin of interfacial droplets with one-step adhesives. J Dent Res. 2007. 86:739–744.
Article38. Maciel KT, Carvalho RM, Ringle RD, Preston CD, Russell CM, Pashley DH. The effect of acetone, ethanol, HEMA, air on the stiffness of human decalcified dentin matrix. J Dent Res. 1996. 75:1851–1858.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of solvent volatilization time on the bond strength of etch-and-rinse adhesive to dentin using conventional or deproteinization bonding techniques
- The effect of priming etched dentin with solvent on the microtensile bond strength of hydrophobic dentin adhesive
- The effect of various bonding systems on the microtensile bond strength of immediate and delayed dentin sealing
- Microleakage of dentin bonding agents in porcelain laminate veneer under simulated physiologic pressure
- Effect of an intermediate bonding resin and flowable resin on the compatibility of two-step total etching adhesives with a self-curing composite resin