J Breast Cancer.
2015 Dec;18(4):394-399. 10.4048/jbc.2015.18.4.394.
Histologic Grade and Decrease in Tumor Dimensions Affect Axillary Lymph Node Status after Neoadjuvant Chemotherapy in Breast Cancer Patients
- Affiliations
-
- 1Department of Radiology, Ajou University School of Medicine, Suwon, Korea.
- 2Department of Surgery, Ajou University School of Medicine, Suwon, Korea. smartblade@gmail.com
- KMID: 2176290
- DOI: http://doi.org/10.4048/jbc.2015.18.4.394
Abstract
- PURPOSE
The purposes our study was to find out any histologic factors associated with negative conversion of axillary lymph node (ALN) after neoadjuvant chemotherapy (NAC). We also evaluated the association between the decrease in size of primary breast tumor and negative conversion of ALN.
METHODS
From January 2012 to November 2014, we included 133 breast cancer patients who underwent NAC and who had ALN metastases which were confirmed on fine-needle aspiration or core needle biopsy at initial diagnosis. All 133 patients underwent initial magnetic resonance imaging (MRI) at the time of diagnosis and preoperative MRI after completion of NAC. We measured the longest dimension of primary breast cancer on MRI.
RESULTS
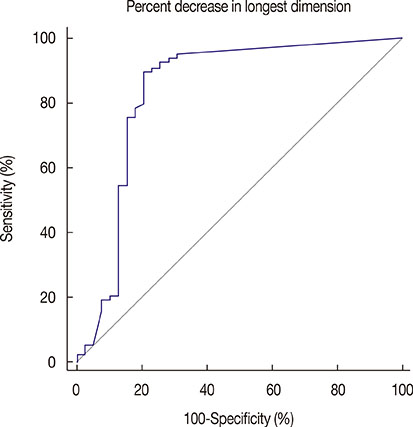
Of 133 patients, 39 patients (29%) showed negative conversion of ALN and of these 39 patients, 25 patients (64%) showed pathologic complete remission of primary breast. On univariate analysis, mean percent decrease in longest dimension, estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 status and histologic grade were significantly associated with the ALN status after NAC (p<0.001, p=0.001, p< 0.001, p=0.001, p=0.002, respectively). On multivariate logistic regression analysis, percent decrease in longest dimension (odds ratio, 1.026; 95% confidence interval [CI], 1.009-1.044) and histologic grade (odds ratio, 3.964; 95% CI, 1.151-13.657) were identified as being independently associated with the ALN status after NAC. The area under the receiver operating characteristic curve was 0.835 with the best cutoff value of 80% decrease in longest dimension. Combination of high histologic grade and more than 80% decrease in longest dimension showed 64% sensitivity and 92% specificity.
CONCLUSION
High histologic grade and more than 80% decrease in primary tumor dimension were associated with negative conversion of ALN after NAC.
MeSH Terms
-
Biopsy, Fine-Needle
Biopsy, Large-Core Needle
Breast Neoplasms*
Breast*
Diagnosis
Drug Therapy*
Estrogens
Humans
Logistic Models
Lymph Nodes*
Magnetic Resonance Imaging
Neoplasm Metastasis
Receptor, Epidermal Growth Factor
Receptors, Progesterone
ROC Curve
Sensitivity and Specificity
Estrogens
Receptor, Epidermal Growth Factor
Receptors, Progesterone
Figure
Reference
-
1. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006; 98:599–609.
Article2. Cox CE, Bass SS, Ku NN, Berman C, Shons AR, Yeatman TJ, et al. Sentinel lymphadenectomy: a safe answer to less axillary surgery? Recent Results Cancer Res. 1998; 152:170–179.
Article3. Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007; 25:3657–3663.
Article4. Ju NR, Jeffe DB, Keune J, Aft R. Patient and tumor characteristics associated with breast cancer recurrence after complete pathological response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013; 137:195–201.
Article5. Gonzalez-Angulo AM, McGuire SE, Buchholz TA, Tucker SL, Kuerer HM, Rouzier R, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol. 2005; 23:7098–7104.
Article6. Tanioka M, Shimizu C, Yonemori K, Yoshimura K, Tamura K, Kouno T, et al. Predictors of recurrence in breast cancer patients with a pathologic complete response after neoadjuvant chemotherapy. Br J Cancer. 2010; 103:297–302.
Article7. Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003; 21:4165–4174.
Article8. Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003; 21:2600–2608.
Article9. Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002; 20:1456–1466.
Article10. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–2027.
Article11. Zhang GC, Liao N, Guo ZB, Qian XK, Ren CY, Yao M, et al. Accuracy and axilla sparing potentials of sentinel lymph node biopsy with methylene blue alone performed before versus after neoadjuvant chemotherapy in breast cancer: a single institution experience. Clin Transl Oncol. 2013; 15:79–84.
Article12. Kuerer HM, Newman LA, Buzdar AU, Hunt KK, Dhingra K, Buchholz TA, et al. Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg. 1998; 176:502–509.
Article13. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.
Article14. Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002; 20:1304–1310.
Article15. Balu-Maestro C, Chapellier C, Bleuse A, Chanalet I, Chauvel C, Largillier R. Imaging in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. Breast Cancer Res Treat. 2002; 72:145–152.
Article16. Rosen EL, Blackwell KL, Baker JA, Soo MS, Bentley RC, Yu D, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003; 181:1275–1282.
Article17. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013; 14:609–618.
Article18. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–1461.
Article19. Charfare H, Limongelli S, Purushotham AD. Neoadjuvant chemotherapy in breast cancer. Br J Surg. 2005; 92:14–23.
Article20. Brown AS, Hunt KK, Shen J, Huo L, Babiera GV, Ross MI, et al. Histologic changes associated with false-negative sentinel lymph nodes after preoperative chemotherapy in patients with confirmed lymph nodepositive breast cancer before treatment. Cancer. 2010; 116:2878–2883.
Article21. Park S, Park JM, Cho JH, Park HS, Kim SI, Park BW. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with cytologically proven node-positive breast cancer at diagnosis. Ann Surg Oncol. 2013; 20:2858–2865.
Article22. Hieken TJ, Boughey JC, Jones KN, Shah SS, Glazebrook KN. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann Surg Oncol. 2013; 20:3199–3204.
Article23. Schipper RJ, Moossdorff M, Beets-Tan RG, Smidt ML, Lobbes MB. Noninvasive nodal restaging in clinically node positive breast cancer patients after neoadjuvant systemic therapy: a systematic review. Eur J Radiol. 2015; 84:41–47.
Article24. You S, Kang DK, Jung YS, An YS, Jeon GS, Kim TH. Evaluation of lymph node status after neoadjuvant chemotherapy in breast cancer patients: comparison of diagnostic performance of ultrasound, MRI and (18)F-FDG PET/CT. Br J Radiol. 2015; 88:20150143.25. Boughey JC, Ballman KV, Hunt KK, McCall LM, Mittendorf EA, Ahrendt GM, et al. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the American College of Surgeons Oncology Group Z1071 Trial (Alliance). J Clin Oncol. 2015; 33:3386–3393.
Article26. Nagashima T, Sakakibara M, Kadowaki M, Suzuki TH, Yokomizo J, Ohki Y, et al. Response rate to neoadjuvant chemotherapy measured on imaging predicts early recurrence and death in breast cancer patients with lymph node involvements. Acta Radiol. 2011; 52:241–246.
Article27. Kuerer HM, Newman LA, Buzdar AU, Dhingra K, Hunt KK, Buchholz TA, et al. Pathologic tumor response in the breast following neoadjuvant chemotherapy predicts axillary lymph node status. Cancer J Sci Am. 1998; 4:230–236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Predictive Factors Affecting Axillary Lymph Node Metastasis in Patients with Invasive Breast Carcinoma of 1 cm or Less
- The Prediction of Axillary Lymph Node Metastasis in T1 Breast Cancer
- Predictors of Axillary Lymph Node Metastases in Patients with T1 Breast Cancer
- Neoadjuvant Chemotherapy Decreases the Identification Rate of Sentinel Lymph Node Biopsy
- The Predicition of Axillary Lymph Node Metastasis in T1 Breast Cancer