J Korean Acad Conserv Dent.
2008 Jul;33(4):332-340. 10.5395/JKACD.2008.33.4.332.
Evaluation of the viability of periodontal ligament cell in rat teeth using slow cryopreservation method with magnetic field
- Affiliations
-
- 1Department of Conservative Dentistry, Yonsei University, Seoul, Korea. andyendo@yuhs.ac
- KMID: 2176001
- DOI: http://doi.org/10.5395/JKACD.2008.33.4.332
Abstract
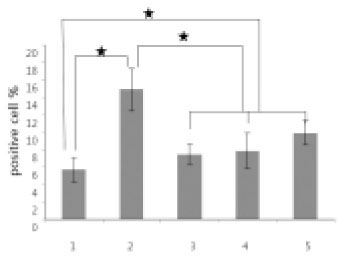
- The purpose of this study was to evaluate the viability of periodontal ligament cell in rat teeth using slow cryopreservation method with magnetic field through MTT assay and TUNEL test. For each group, 12 teeth of 4 weeks old white female Sprague-Dawley rat were used for MTT assay, and 6 teeth in TUNEL test. The Maxillary left and right, first and second molars were extracted as atraumatically as possible under tiletamine anesthesia. The experimental groups were group1 (immediately extraction), group 2 (cold preservation at 4degrees C for 1 week), group 3 (rapid cryopreservation in liquid nitrogen), group 4 (slow cryopreservation with magnetic field of 1 G), and group 5 (slow cryopreservation). F medium was used as preservation medium and 10% DMSO as cryoprotectant. After preservation and thawing, the MTT assay and TUNEL test were processed. One way ANOVA and Scheffe method were performed at the 95% level of confidence. The value of optical density obtained after MTT analysis was divided by the value of eosin staining for tissue volume standardization. In both MTT assay and TUNEL test, it had showed no significant difference among group 3, 4, and 5. And group 3 had showed higher viability of periodontal ligament cell than group 2. From this study, slow cryopreservation method with magnetic field can be used as one of cryopreservation methods.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Comparison of viability of oral epithelial cells stored by different freezing methods
Do-Young Baek, Seung-Jong Lee, Han-Sung Jung, EuiSeong Kim
J Korean Acad Conserv Dent. 2009;34(6):491-499. doi: 10.5395/JKACD.2009.34.6.491.The evaluation of periodontal ligament cells of rat teeth after low-temperature preservation under high pressure
Jin-Ho Chung, Jin Kim, Seong-Ho Choi, Eui-Seong Kim, Jiyong Park, Seung-Jong Lee
J Korean Acad Conserv Dent. 2010;35(4):285-294. doi: 10.5395/JKACD.2010.35.4.285.
Reference
-
1. Kristerson L, Johansson LA, Kisch J, Stadler LE. Autotransplantation of third molars as treatment in advanced periodontal disease. J Clin Periodontol. 1991. 18:521–528.
Article2. Schwartz O. Cryopreservation as long-term storage of teeth for transplantation or replantation. Int J Oral Maxillofac Surg. 1986. 15:30–32.
Article3. Rubinsky B. Principles of low temperature cell preservation. Heart Fail Rev. 2003. 8:277–284.4. Lovelock JE, Polge C. The immobilization of spermatozoa by freezing and thawing and the protective action of glycerol. Biochem J. 1954. 58:618–622.
Article5. Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984. 247:C125–C142.
Article6. Kawasaki N, Hamamoto Y, Nakajima T, Irie K, Ozawa H. Periodontal regeneration of transplanted rat molars after cryopreservation. Arch Oral Biol. 2004. 49:59–69.
Article7. Kawata T. Tooth transplantation by teeth bank-approach to human-Hiroshima. 2005. Department of Orthodontics, Hiroshima University School of Dentistry;Thesis.8. Kim ES, Jeon IS, Kim JW, Kim J, Jung HS, Lee SJ. An MTT-based method for quantification of periodontal ligament cell viability. Oral Dis. 2007. 13:495–499.
Article9. Schwartz O, Andreasen JO, Greve T. Cryopreservation before replantation of mature teeth in monkeys(II). Effect of preincubation, different freezing and equilibration rates and endodontic treatment upon periodontal healing. Int J Oral Surg. 1985. 14:350–361.10. Ashwood-Smith MJ. Low temperature preservation of cells, tissues and organs. 1980. Pitman Medical;19–45.11. Stuart M. Wentworth. Fundamentals of electromagnetics with engineering application. 2004. 2nd ed. Chicago: Science & Technology;156–160.12. Marsland TP, Evans S, Pegg DE. Dielectric measurements for the design of an electromagnetic rewarming system. Cryobiology. 1987. 24:311–323.
Article13. Wusteman M, Robinson M, Pegg D. Vitrification of large tissues with dielectric warming: biological problems and some approached to their solution. Cryobiology. 2004. 48:179–189.
Article14. Kaku M, Kamata H, Kawata T. Cryopreservation of PDL cells by use of program freezer with magnetic field for tooth banking. Dent Jpn (Tokyo). 2007. 43:82–86.15. RAPID Report. http://www.niehs.nih.gov/emfrapid/home.htm.16. Jeon IS. Evaluation of periodontal ligament cell viability in rat teeth according to various extra-oral dry time using MTT assay and a histologic verification. 2004. Yonsei University, Graduate School;PhD Thesis.17. Oh YH, Che ZM, Hong JC, Lee EJ, Lee SJ, Kim J. Cryopreservation of human teeth for future organization of a tooth bank-a preliminary study. Cryobiology. 2005. 51:322–329.
Article18. Ashkenazi M, Sarnat H, Keila S. In vitro viability, mitogenicity and clonogenic capacity of periodontal ligament cells after storage in six different media. Endod Dent Traumatol. 1999. 15:149–156.
Article19. Fischer S, Maclean AA, Liu M, Cardella JA, Slutsky AS, Suga M, Moreira JF, Keshavjee S. Dynamic changes in apoptotic and necrotic cell death correlate with severity of ischemia-reperfusion injury in lung transplantation. Am J Respir Crit Care Med. 2000. 162:1932–1939.
Article20. Kerr JF. History of the events leading to the formulation of the apoptosis concept. Toxicology. 2002. 181-182:471–474.
Article21. Park SY, Kim EY, Cui XS, Tae JC, Lee WD, Kim NH, Park SP, Lim JH. Increase in DNA fragmentation and apoptosis-related gene expression in frozen-thawed bovine blastocysts. Zygote. 2006. 14:125–131.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The evaluation of periodontal ligament cells of rat teeth after low-temperature preservation under high pressure
- Evaluation of vitrification for cryopreservation of teeth
- The efficacy of programmed cryo-preservation under pressure in rat periodontal ligament cells
- Effects of Slow Programmable Cryopreservation on Preserving Viability of the Cultured Periodontal Ligament Cells from Human Impacted Third Molar
- Evaluation of periodontal ligament cell viability in rat teeth after frozen preservation using in-vivo MTT assay