Chonnam Med J.
2008 Dec;44(3):151-156. 10.4068/cmj.2008.44.3.151.
Long Term Follow-up Results of Topical 0.05% Cyclosporine A in Patient with Dry Eye
- Affiliations
-
- 1Department of Ophthalmology, Chonnam National University Medical School, Gwangju, Korea.
- 2Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Ophthalmology, Ulsan Hospital, College of Medicine, Ulsan University, Ulsan, Korea.
- 4Chonnam National University Research Institute of Medical Sciences, Gwangju, Korea. kcyoon@jnu.ac.kr
- KMID: 2172294
- DOI: http://doi.org/10.4068/cmj.2008.44.3.151
Abstract
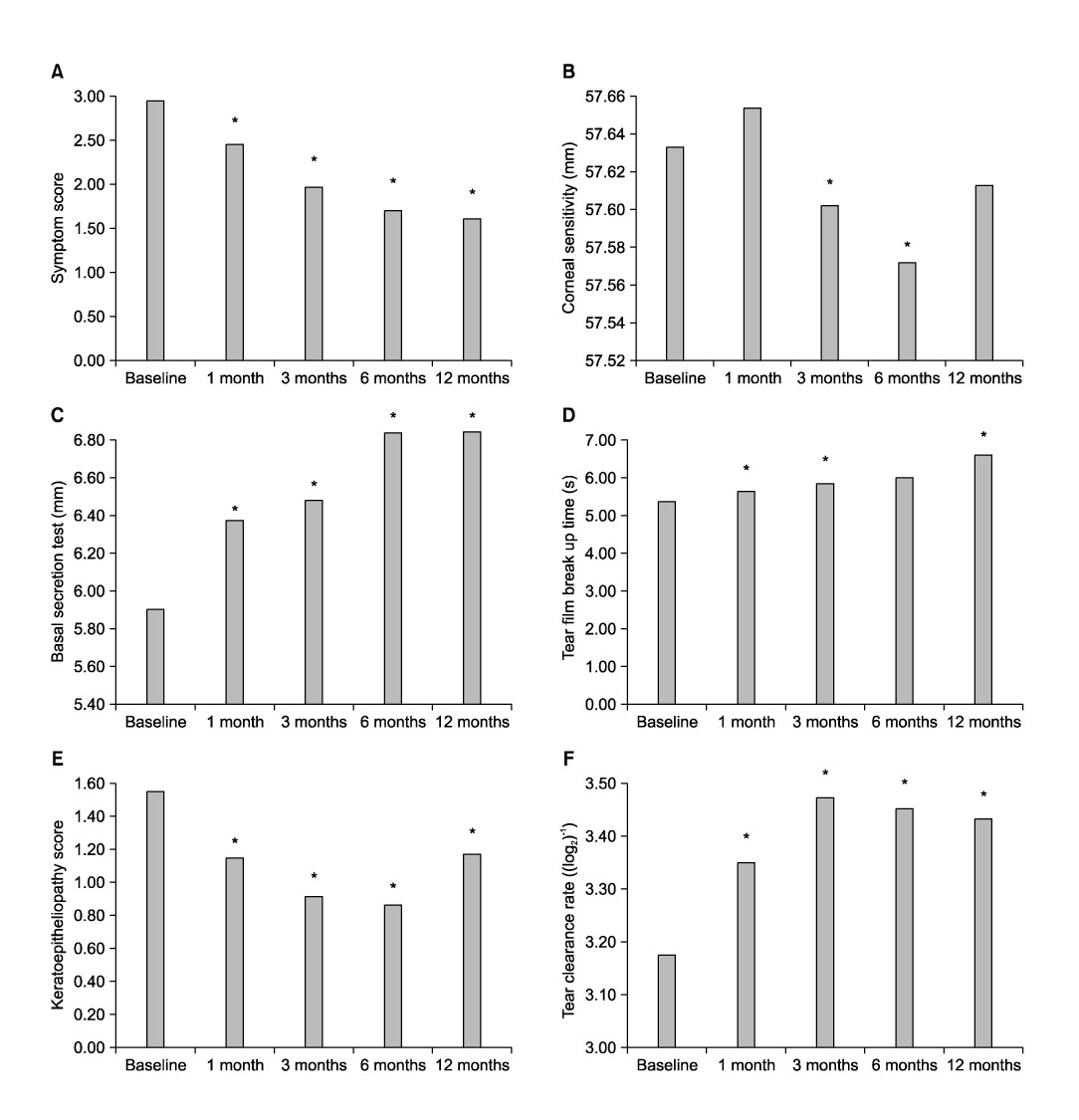
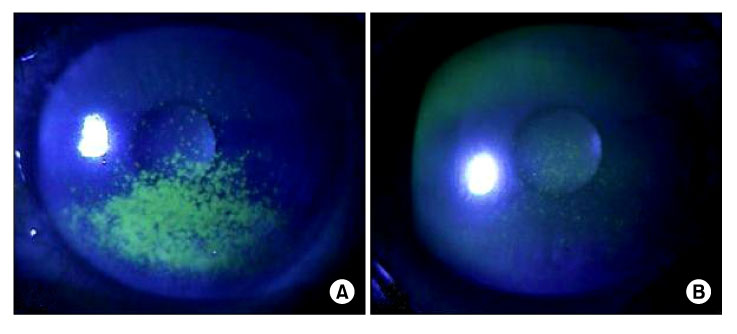
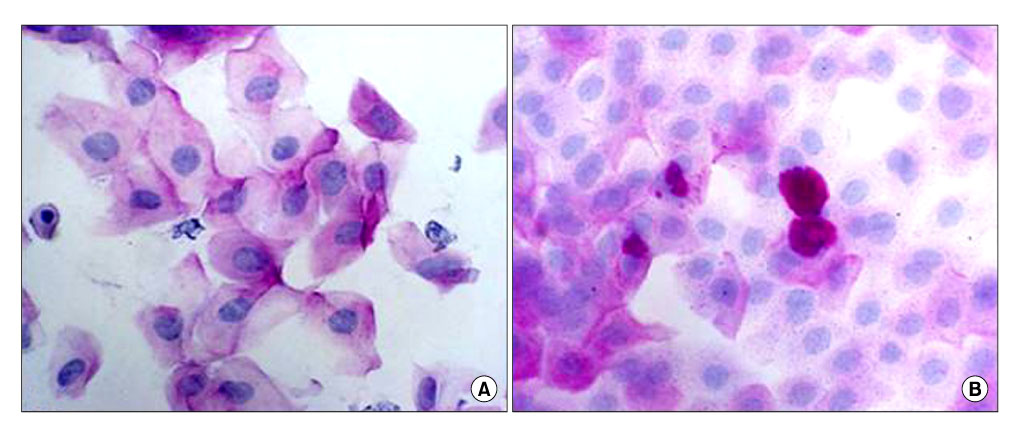
- To evaluate the therapeutic effects of 0.05% cylosporine A eyedrops (Restasis, Allergan Inc., Irvine, CA, USA.) in patients with dry eye syndrome, one hundred thirty-five patients (270 eyes) with dry eye syndrome were treated with 0.05% cylosporine A (CsA) and lubrication eyedrops for 12 months. Before and 1, 3, 6 and 12 months after treatment, symptom score, corneal sensitivity, tear film break-up time (BUT), keratoepitheliopathy score, basal tear secretion, tear clearance rate were measured. Before and 3 months after treatment, conjunctival impression cytology were done. One month after treatment, symptom score (2.38+/-0.80), basal tear secretion (6.34+/-3.26 mm), and keratoepitheliopathy score (1.10+/-1.54) were improved from 2.87+/-0.62 (p<0.01), 5.89+/-2.72 mm (p<0.01) and 1.50+/-1.82 (p<0.01), respectively. Three months after treatment, tear film break-up time (5.70+/-1.76 sec) and tear clearance test (13.52+/-11.0) were improved from 5.24+/-2.01 sec (p=0.01) and 10.74+/-8.48 (p=0.02), respectively. Six months after treatment, corneal sensitivity were improved from 57.85+/-4.27 mm to 57.57+/-6.24 mm (p=0.01) during the follow up period. Treatment of dry eye syndrome with topical CsA resulted in an increase in goblet cell numbers and decrease in conjunctival keratinization. Fourteen patients (10.4%) were discontinued instillation of topical CsA due to burning sensation and pain. Use of 0.05% topical CsA emulsion is effective for dry eye syndrome in helping to improve ocular symptom, and parameters of tear film and ocular surface during the follow-up period of 12 months.
MeSH Terms
Figure
Cited by 1 articles
-
Clinical Efficacy of Topical 3% Diquafosol Tetrasodium in Short Tear Film Break-Up Time Dry Eye
Hyun Ho Jung, Yeon Soo Kang, Mi Sun Sung, Kyung Chul Yoon
J Korean Ophthalmol Soc. 2015;56(3):339-344. doi: 10.3341/jkos.2015.56.3.339.
Reference
-
1. Tang-Liu DD, Acheampong A. Ocular pharmacokinetics and safety of ciclosporin, a novel topical treatment for dry eye. Clin Pharmacokinet. 2005. 44:247–261.
Article2. Dick AD, Azim M, Forrester JV. Immunosuppressive therapy for chronic uveitis: optimising therapy with steroids and cyclosporin A. Br J Ophthalmol. 1997. 81:1107–1112.
Article3. Stevenson D, Tauber J, Reis BL. The Cyclosporin A Phase 2 Study Group. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. Ophthalmology. 2000. 107:967–974.
Article4. Miyata K, Amano S, Sawa M, Nishida T. A novel grading method for superficial punctate keratopathy magnitude and its correlation with cornea epithelial permeability. Arch Ophthalmol. 2003. 121:1537–1539.
Article5. Anshu , Munshi MM, Sathe V, Ganar A. Conjunctival impression cytology in contact lens wearers. Cytopathology. 2001. 12:314–320.
Article6. Nelson JD. Impression cytology. Cornea. 1988. 7:71–81.
Article7. Yoon KC, Heo H, Im SK, You IC, Kim YH, Park YG. Comparison of autologous serum and umbilical cord serum eyedrops for dry eye syndrome. Am J Ophthalmol. 2007. 144:86–92.8. Dick AD, Azim M, Forrester JV. Immunosuppressive therapy for chronic uveitis: optimising therapy with steroids and cyclosporin A. Br J Ophthalmol. 1997. 81:1107–1112.
Article9. Tatlipinar S, Akpek EK. Topical ciclosporin in the treatment of ocular surface disorders. Br J Ophthalmol. 2005. 89:1363–1367.
Article10. Her J, Yu SI, Seo SG. Clinical effects of various antiinflammatory therapies in dry eye syndrome. J Korean Ophthalmol Soc. 2006. 47:1901–1910.11. Kaan G, özden ö. Therapeutic use of topical cyclosporine. Ann Ophthalmol. 1993. 25:182–186.12. Turner K, Pflugfelder SC, Ji Z, Feuer WJ, Stern M, Reis BL. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000. 19:492–496.
Article13. Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005. 24:80–85.
Article14. Sall K, Stevenson OD, Mundorf TK, Reis BL. CsA Phase 3 Study Group. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000. 107:631–639.
Article15. Perry HD, Doshi-Carnevale S, Donnenfeld ED, Solomon R, Biser SA, Bloom AH. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea. 2006. 25:171–175.
Article16. Kinoshita S, Kiorpes TC, Friend J, Thoft RA. Goblet cell density in ocular surface disease. A better indicator than tear mucin. Arch Ophthalmol. 1983. 101:1284–1287.17. Barber LD, Pflugfelder SC, Tauber J, Foulks GN. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology. 2005. 112:1790–1794.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Evaluation After Topical Cyclosporine Treatment in Dry Eye Patients With Graft-Versus-Host Disease
- The Effect of Topical Cyclosporine 0.05% on Dry Eye after Cataract Surgery
- Effect of Sodium Hyaluronate and Cyclosporine A on Tear Film in Dry Eye Syndrome
- Efficacy of Topical Cyclosporine in Mild Dry Eye Patients Having Refractive Surgery
- Study of Utilization Pattern and Compliance with Topical 0.05% Cyclosporine Emulsion in Korean Dry Eye Patients