Korean J Ophthalmol.
2013 Jun;27(3):167-171. 10.3341/kjo.2013.27.3.167.
The Effect of Topical Cyclosporine 0.05% on Dry Eye after Cataract Surgery
- Affiliations
-
- 1Department of Ophthalmology, Yeouido St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. eyedoc@catholic.ac.kr
- KMID: 1798053
- DOI: http://doi.org/10.3341/kjo.2013.27.3.167
Abstract
- PURPOSE
To evaluate the effectiveness of cyclosporine 0.05% for dry eye after cataract surgery.
METHODS
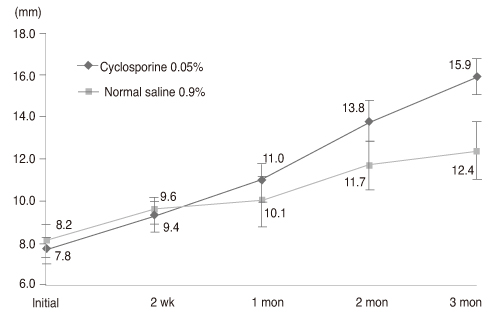
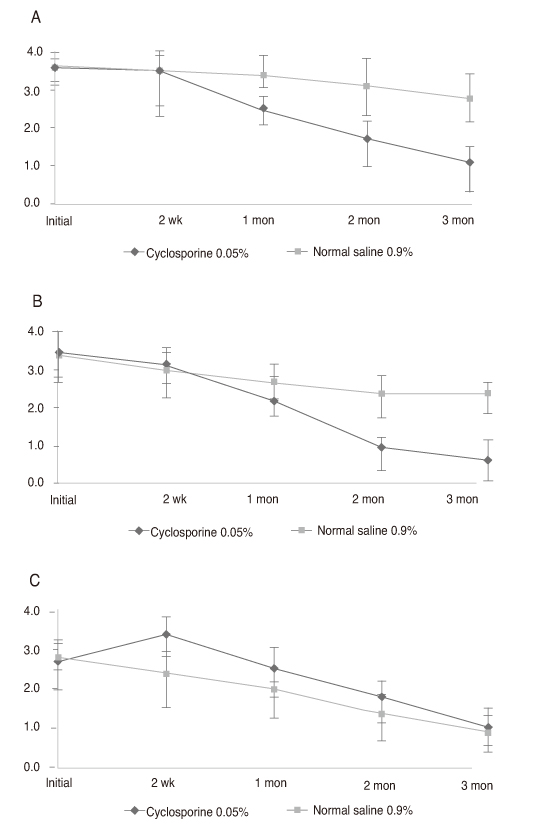
Thirty-two newly diagnosed patients with dry eye syndrome 1 week after cataract surgery received a twice-daily treatment of cyclosporine 0.05% for one eye and normal saline 0.9% for the other. Disease severity was measured at 2 weeks, 1 month, 2 months, and 3 months by Schirmer test I (ST-I), tear film break-up time (tBUT), corneal temperature and dry eye symptom questionnaire (Ocular Surface Disease Index).
RESULTS
Both groups increased in ST-I and tBUT over time. ST-I in the cyclosporine 0.05% group showed a significant increase at 3 months and tBUT in the cyclosporine 0.05% group showed an increase at 2 and 3 months. The dry eye symptom score was significantly reduced in the cyclosporine 0.05% group.
CONCLUSIONS
Cyclosporine 0.05% can also be an effective treatment for dry eye after cataract surgery.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
*Cataract Extraction
Cyclosporine/*administration & dosage
Dry Eye Syndromes/*drug therapy
Female
Humans
Immunosuppressive Agents/administration & dosage
Male
Middle Aged
Ophthalmic Solutions/*administration & dosage
Postoperative Complications/*drug therapy
Treatment Outcome
Immunosuppressive Agents
Ophthalmic Solutions
Cyclosporine
Figure
Cited by 2 articles
-
Effectiveness of Cyclosporine-steroid Treatment after Cataract Surgery according to Dry Eye Severity
Jae Yeong Park, Sang Cheol Yang, Young Min Park, Ji Eun Lee, Choul Yong Park, Jong Soo Lee
J Korean Ophthalmol Soc. 2019;60(9):821-828. doi: 10.3341/jkos.2019.60.9.821.Effect of Cyclosporin A on Tear Film and Corneal Aberration after Cataract Surgery
Jei Hun Jeon, Hong Seok Kim, Ji Won Jung, Sang Chul Yoon, Kyoung Yul Seo, Hyung Keun Lee, Eung Kweon Kim, Tae Im Kim
J Korean Ophthalmol Soc. 2014;55(7):978-983. doi: 10.3341/jkos.2014.55.7.978.
Reference
-
1. Kohlhaas M. Corneal sensation after cataract and refractive surgery. J Cataract Refract Surg. 1998. 24:1399–1409.2. Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007. 26:9 Suppl 1. S16–S20.3. Cho YK, Kim MS. Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol. 2009. 23:65–73.4. Wilson SE, Perry HD. Long-term resolution of chronic dry eye symptoms and signs after topical cyclosporine treatment. Ophthalmology. 2007. 114:76–79.5. Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009. 54:321–338.6. Thomas PB, Samant DM, Zhu Z, et al. Long-term topical cyclosporine treatment improves tear production and reduces keratoconjunctivitis in rabbits with induced autoimmune dacryoadenitis. J Ocul Pharmacol Ther. 2009. 25:285–292.7. Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004. 137:337–342.8. Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004. 78:409–416.9. Donnenfeld ED, Solomon K, Perry HD, et al. The effect of hinge position on corneal sensation and dry eye after LASIK. Ophthalmology. 2003. 110:1023–1029.10. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007. 5:75–92.11. Walker TD. Benzalkonium toxicity. Clin Experiment Ophthalmol. 2004. 32:657.12. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000. 107:631–639.13. Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000. 47:119–125.14. Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002. 43:2609–2614.15. Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005. 24:80–85.16. Sahli E, Hoşal BM, Zilelioglu G, et al. The effect of topical cyclosporine A on clinical findings and cytological grade of the disease in patients with dry eye. Cornea. 2010. 29:1412–1416.17. Baiza-Durán L, Medrano-Palafox J, Hernandez-Quintela E, et al. A comparative clinical trial of the efficacy of two different aqueous solutions of cyclosporine for the treatment of moderate-to-severe dry eye syndrome. Br J Ophthalmol. 2010. 94:1312–1315.18. Tananuvat N, Daniell M, Sullivan LJ, et al. Controlled study of the use of autologous serum in dry eye patients. Cornea. 2001. 20:802–806.19. Mengher LS, Pandher KS, Bron AJ, Davey CC. Effect of sodium hyaluronate (0.1%) on break-up time (NIBUT) in patients with dry eyes. Br J Ophthalmol. 1986. 70:442–447.20. Sheppard JD, Scoper SV, Samudre S. Topical loteprednol pretreatment reduces cyclosporine stinging in chronic dry eye disease. J Ocul Pharmacol Ther. 2011. 27:23–27.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of 0.1% Sodium Hyaluronate and 0.05% Cyclosporine on Tear Film Parameters after Cataract Surgery
- Comparison of the Effects of 0.05% and 0.1% Cyclosporine for Dry Eye Syndrome Patients after Cataract Surgery
- Effectiveness of Cyclosporine-steroid Treatment after Cataract Surgery according to Dry Eye Severity
- The Effect of Topical Cyclosporine 0.05% on Tear Osmolarity for Dry Eye Syndrome
- Efficacy of Topical Cyclosporine in Mild Dry Eye Patients Having Refractive Surgery