Chonnam Med J.
2010 Aug;46(2):105-111. 10.4068/cmj.2010.46.2.105.
Study of Utilization Pattern and Compliance with Topical 0.05% Cyclosporine Emulsion in Korean Dry Eye Patients
- Affiliations
-
- 1Department of Ophthalmology, Chonnam National University Medical School and Hospital, Gwangju, Korea. kcyoon@chonnam.ac.kr
- 2Department of Ophthalmology, Chonbuk National University Medical School and Hospital, Jeonju, Korea.
- KMID: 1730007
- DOI: http://doi.org/10.4068/cmj.2010.46.2.105
Abstract
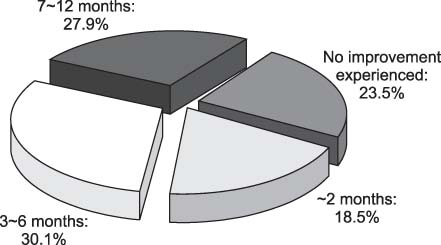
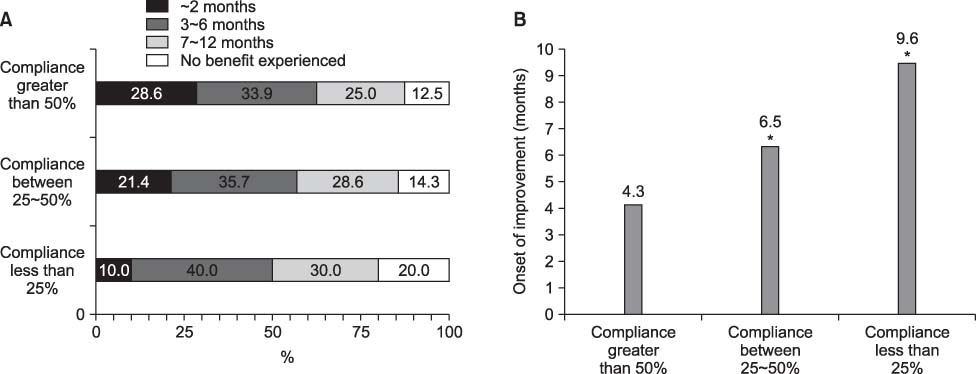
- We investigate the utilization pattern and self-reported compliance with topical 0.05% cyclosporine emulsion in patients with dry eye. In 136 patients with dry eye using 0.05% cyclosporine emulsion, the level of compliance, the symptoms before and after use, the length of time taken before symptoms improved and adverse effects when use were evaluated. Eighty two percent of 136 patients used at least 1 box of 0.05% cyclosporine emulsion. One hundred and sixty patients were divided into three groups. Patients whose compliance level was higher than 50% were assigned to Group I; those with a compliance level between 25 to 50% belonged to Group II and those whose compliance was less than 25% were in Group III. The symptom score of all subjects decreased from 2.96 before cyclosporine use to 1.72 (p=0.011) after cyclosporine use. Patients in Group I experienced an improvement in their symptoms earlier (4.3 months) compared to those in Group II (6.5 months, p=0.030) and Group III (9.6 months, p=0.010). Adverse effects were experienced in 60% of patients, the most common being a sensation of pain (20%) and burning (20%). Patients who had the highest level of compliance noticed an improvement in their symptoms earliest. Patients need to be given detailed explanations about the adverse events of 0.05% cyclosporine emulsion.
Keyword
Figure
Reference
-
1. Darsun D, Kim MC, Solomon A, Pflugfelder SC. Treatment of recalcitrant recurrent corneal epithelial erosions with inhibitors of matrix metalloproteinases-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001. 132:8–13.2. Marsh P, Pflugfelder SC. Topical nonpreserved methylpredni solone-plus therapy of keratoconjunctivitis sicca in Sjögren's syndrome. Ophthalmology. 1999. 106:509–512.3. Gündüz K, Ozdemir O. Topical cyclosporin treatment of keratoconjunctivitis sicca in secondary Sjögren's syndrome. Acta Ophthalmol (Copenh). 1994. 72:438–442.
Article4. Tsubota K, Goto E, Fujita H, Ono M, Inoue H, Saito I, et al. Treatment of dry eye by autologous serum application in Sjögren's syndrome. Br J Ophthalmol. 1999. 83:390–395.
Article5. Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000. 19:644–649.6. Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998. 17:584–589.7. Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004. 78:409–416.
Article8. Smith VA, Rishmawi H, Hussein H, Easty DL. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001. 85:147–153.
Article9. Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001. 42:2283–2292.10. Argüeso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. 2002. 43:1004–1011.11. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002. 120:330–337.
Article12. Sall K, Stevenson OD, Mundorf TK, Reis BL. CsA Phase 3 Study Group. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000. 107:631–639.
Article13. Stevenson D, Tauber J, Reis BL. The Cyclosporine A Phase 2 Study Group. Efficacy and safety of cyclosporine A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. Ophthalmology. 2000. 107:967–974.
Article14. Brignole F, Pisella PJ, De Saint Jean M, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporine A. Invest Ophthalmol Vis Sci. 2001. 42:90–95.15. Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome:effect on conjunctival lymphocytes. Arch Ophthalmol. 2000. 118:1489–1496.
Article16. Turner K, Pflugfelder SC, Ji Z, Feuer WJ, Stern M, Reis BL. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000. 19:492–496.
Article17. Ozcan AA, Ersoz TR, Dulger E. Management of severe allergic conjunctivitis with topical cyclosporine A 005% eyedrops. Cornea. 2007. 26:1035–1038.
Article18. Kiliç A, Gürler B. Topical 2% cyclosporine A in preservative free artificial tears for the treatment of vernal keratoconjunctivitis. Can J Ophthalmol. 2006. 41:693–698.
Article19. Reinhard T, Sundmacher R. Local cyclosporin A therapy in Thygeson superficial punctate keratitis--a pilot study. Klin Monatsbl Augenheilkd. 1996. 209:224–227.
Article20. Sahin A, Bozkurt B, Irkec M. Topical cyclosporine a in the treatment of superior limbic keratoconjunctivitis: a long-term follow-up. Cornea. 2008. 27:193–195.
Article21. Rubin M, Rao SN. Efficacy of topical cyclosporin 0.05% in the treatment of posterior blepharitis. J Ocul Pharmacol Ther. 2006. 22:47–53.
Article22. Perry HD, Doshi-Carnevale S, Donnenfeld ED, Solomon R, Biser SA, Bloom AH. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea. 2006. 25:171–175.
Article23. Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surgery. 1995. 26:233–236.
Article24. MacKean JM, Elkington AR. Compliance with treatment of patients with chronic open algle glaucoma. Br J Ophthalmol. 1983. 67:46–49.25. Ashburn FS Jr, Goldberg I, Kass MA. Compliance with ocular therapy. Surv Ophthalmol. 1980. 24:237–248.
Article26. Trattler W, Katsev D, Kerney D. Self-reported compliance with topical cyclosporine emulsion 0.05% and onset of the effects of increased tear production as assessed through patient surveys. Clin Ther. 2006. 28:1848–1856.
Article27. Chiang TH, Walt JG, McMahon JP Jr, Mansfield JE Jr, Simonyi S. Real-world utilization patterns of cyclosporine ophthalmic emulsion 0.05% within managed care. Can J Clin Pharmacol. 2007. 14:e240–e245.28. Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004. 137:337–342.
Article29. Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003. 22:7 Suppl. S19–S27.
Article30. Ogawa Y, Okamoto S, Wakui M, Watanabe R, Yamada M, Yoshino M, et al. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol. 1999. 83:1125–1130.
Article31. Nussenblatt RB, Palestine AG. Cyclosporin: immunology, phamacology and therapeutic use. Surv Ophthalmol. 1986. 31:159–169.32. Small DS, Acheampong A, Reis B, Stern K, Stewart W, Berdy G, et al. Blood concentrations of cyclosporin a during long term treatment with cyclosporine a ophthalmic emulsion in patients with moderate to severe dry eye disease. J Ocul Pharmacol Ther. 2002. 18:411–418.
Article33. Sall K, Stevenson OD, Mundorf TK, Reis BL. CsA Phase 3 Study Group. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000. 107:631–639.
Article34. Stevenson D, Tauber J, Reis BL. The Cyclosporin A Phase 2 Study Group. Efficacy and safety of cyclosporin a ophthalmic emulsion in the treatment of moderate to severe dry eye syndrome: a dose-ranging, randomized trial. Ophthalmology. 2000. 107:967–974.
Article35. Barber LD, Pflugfelder SC, Tauber J, Foulks GN. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Am J Ophthalmol. 2005. 112:1790–1794.
Article36. Stonecipher K, Perry HD, Gross RH, Kerney DL. The impact of topical cyclosporine A emulsion 0.05% on the outcomes of patients with keratoconjunctivitis sicca. Curr Med Res Opin. 2005. 21:1057–1063.
Article37. Bigger JF. A comparison of patient compliance in treated vs untreated ocular hypertension. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976. 81:277–285.38. Olander K, Zimmerman TJ. Practical aspects of controlling glaucoma medically or how to make your glaucoma medicines work for you. Glaucoma editorial. Ann Ophthalmol. 1980. 12:717–728.39. Riffenburgh RS. Doctor-patient relationship in glaucoma therapy. Arch Ophthalmol. 1966. 75:204–206.
Article40. Holland EJ, Olsen TW, Ketcham JM, Florine C, Krachmer JH, Purcell JJ, et al. Topical cyclosporin a in the treatment of anterior segment inflammatory disease. Cornea. 1993. 12:413–419.
Article41. Perry HD, Solomon R, Donnenfeld ED, Perry AR, Wittpenn JR, Greenman HE, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol. 2008. 126:1046–1050.
Article42. Kim EC, Choi JS, Joo CK. A comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am J Ophthalmol. 2009. 147:206–213.
Article43. Wang Y, Ogawa Y, Dogru M, Kawai M, Tatematsu Y, Uchino M, et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2008. 41:293–302.
Article44. Brown MM, Brown GC, Brown HC, Peet J, Roth Z. Value-based medicine, comparative effectiveness, and cost-effectiveness analysis of topical cyclosporine for the treatment of dry eye syndrome. Arch Ophthalmol. 2009. 127:146–152.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Affecting Compliance With 0.05% Cyclosporine Emulsion in Patients With Dry Eye Syndrome
- Long-term Evaluation After Topical Cyclosporine Treatment in Dry Eye Patients With Graft-Versus-Host Disease
- The Efficacies and Safeties of a 0.05% Cyclosporine Nanoemulsion and a 0.1% Cyclosporine Cationic Emulsion
- The Effect of Topical Cyclosporine 0.05% on Dry Eye after Cataract Surgery
- Assessment of the Compliance with 0.1% Cyclosporine A in Dry-Eye Patients with Sjögren's Syndrome