Ann Dermatol.
2011 Dec;23(Suppl 3):S360-S363. 10.5021/ad.2011.23.S3.S360.
Pityriasis rosea-like Drug Eruption Induced by Imatinib Mesylate (Gleevec(TM))
- Affiliations
-
- 1Department of Dermatology, College of Medicine, Chungnam National University, Daejeon, Korea. jhoon@cnu.ac.kr
- KMID: 2171872
- DOI: http://doi.org/10.5021/ad.2011.23.S3.S360
Abstract
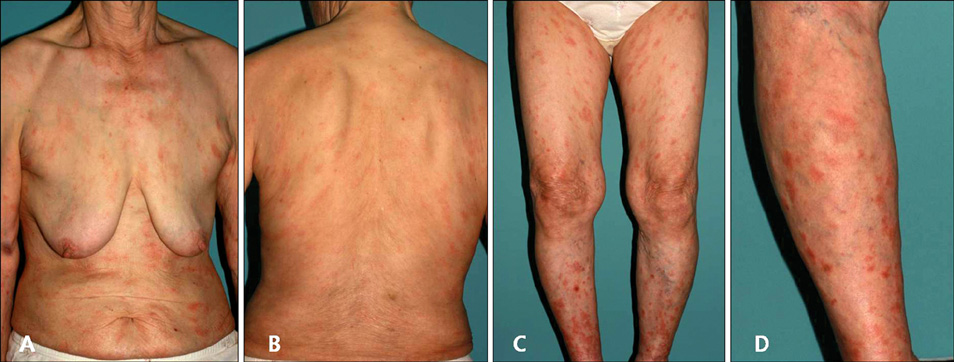
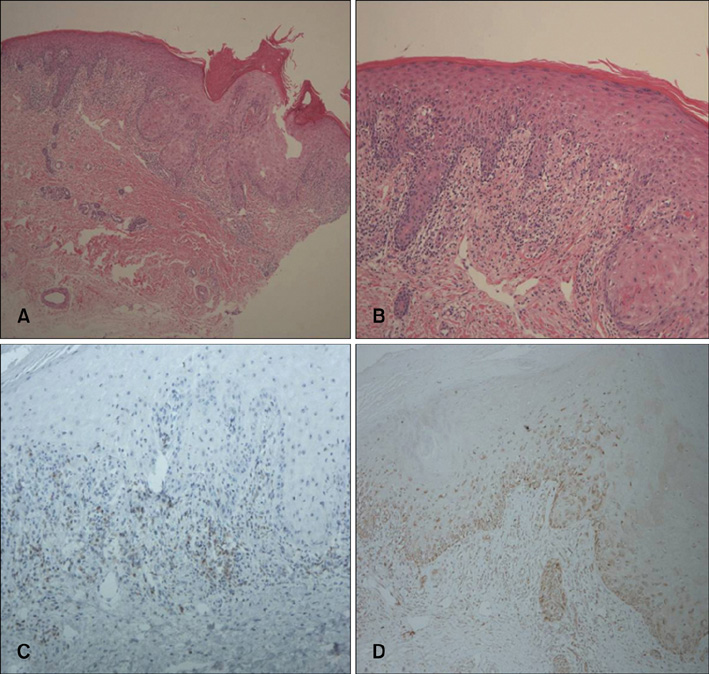
- Imatinib mesylate (Gleevec(TM), STI571), a selective inhibitor of BCR-ABL, c-Kit, and platelet-derived factor receptor, has been used to treat chronic myelogenous leukemia (CML) and gastrointestinal stromal tumors. Although its use has been associated with various adverse cutaneous reactions, pityriasis rosea-like drug eruptions are rare. Here, we report a case of pityriasis rosea-like drug eruption that developed following the administration of imatinib mesylate to treat CML.
Keyword
MeSH Terms
Figure
Reference
-
1. Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001. 344:1031–1037.
Article2. Valeyrie L, Bastuji-Garin S, Revuz J, Bachot N, Wechsler J, Berthaud P, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003. 48:201–206.
Article3. Hsiao LT, Chung HM, Lin JT, Chiou TJ, Liu JH, Fan FS, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002. 117:620–622.
Article4. Brouard MC, Prins C, Mach-Pascual S, Saurat JH. Acute generalized exanthematous pustulosis associated with STI-571 in a patient with chronic myeloid leukemia. Dermatology. 2001. 203:57–59.
Article5. Schwarz M, Kreuzer KA, Baskaynak G, Dörken B, le Coutre P. Imatinib-induced acute generalized exanthematous pustulosis (AGEP) in two patients with chronic myeloid leukemia. Eur J Haematol. 2002. 69:254–256.
Article6. Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001. 344:1038–1042.
Article7. Yoo KH, Rho YK, Kim JY, Li K, Seo SJ, Hong CK. A case of drug eruption with localized exfoliative dermatitis induced by imatinib mesylate. Korean J Dermatol. 2008. 46:1435–1438.8. Joo YH, Min SU, Lee DH, Suh DH. A case of drug eruption with periorbital edema and exfoliative dermatitis induced by imatinib mesylate (STI571, Gleevec(TM)). Korean J Dermatol. 2007. 45:194–196.9. Bae YI, Yun SJ, Lee JB, Kim SJ, Lee SC, Won YH. A case of psoriasiform drug eruption induced by imatinib mesylate (Gleevec™). Korean J Dermatol. 2009. 47:722–725.10. Woo SM, Huh CH, Park KC, Youn SW. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007. 34:724–726.
Article11. Konstantopoulos K, Papadogianni A, Dimopoulou M, Kourelis C, Meletis J. Pityriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002. 205:172–173.
Article12. Brazzelli V, Prestinari F, Roveda E, Barbagallo T, Bellani E, Vassallo C, et al. Pityriasis rosea-like eruption during treatment with imatinib mesylate: description of 3 cases. J Am Acad Dermatol. 2005. 53:5 Suppl 1. S240–S243.
Article13. Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, Gleevec). Dermatology. 2002. 205:169–171.
Article14. Basso FG, Boer CC, Corrêa ME, Torrezan M, Cintra ML, de Magalhães MH, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009. 17:465–468.
Article15. Rozieres A, Vocanson M, Saïd BB, Nosbaum A, Nicolas JF. Role of T cells in nonimmediate allergic drug reactions. Curr Opin Allergy Clin Immunol. 2009. 9:305–310.
Article16. Brouard M, Saurat JH. Cutaneous reactions to STI571. N Engl J Med. 2001. 345:618–619.
Article17. Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, Cordon-Cardo C. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994. 42:1417–1425.
Article18. Scott LC, White JD, Reid R, Cowie F. Management of skin toxicity related to the use of imatinib mesylate (STI571, Glivectrade mark) for advanced stage gastrointestinal stromal tumours. Sarcoma. 2005. 9:157–160.
Article19. Rule SA, O'Brien SG, Crossman LC. Managing cutaneous reactions to imatinib therapy. Blood. 2002. 100:3434–3435.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imatinib Mesylate (Gleevec(TM))-induced Lichenoid Drug Eruption Improved by Tentative Dose-reduction and Topical Steroid
- A Case of Lichenoid Drug Eruption Associated with Imatinib Mesylate
- Comments to "A Case of Pityriasis Rosea Associated with Leuprolide Acetate"
- A Case of Drug Eruption with Periorbital Edema and Exfoliative Dermatitis Induced by Imatinib Mesylate (STI571, Gleevec(TM))
- A Case of Generalized Keratosis Pilaris Induced by Imatinib Mesylate