Ann Dermatol.
2013 May;25(2):203-207. 10.5021/ad.2013.25.2.203.
Merkel Cell Polyomavirus Is Frequently Detected in Korean Patients with Merkel Cell Carcinoma
- Affiliations
-
- 1Department of Dermatology, Chonnam National University Medical School, Gwangju, Korea. jbmlee@jnu.ac.kr
- KMID: 2171789
- DOI: http://doi.org/10.5021/ad.2013.25.2.203
Abstract
- BACKGROUND
Merkel cell carcinoma (MCC) is an increasingly common neuroendocrine cancer of the skin. Merkel cell polyomavirus (MCPyV) is one of the causative agents of MCC. The prevalence of MCPyV in primary MCC and sun-exposed non-MCC tumors has been known to have different results depending on where it was investigated.
OBJECTIVE
This study assesses the prevalence of MCPyV from primary MCC and sun-exposed non-MCC tumors in Korea.
METHODS
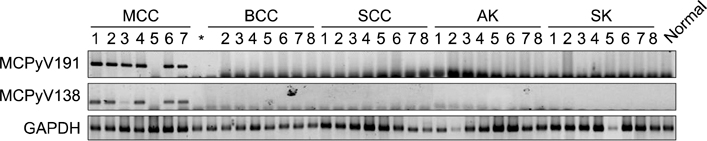
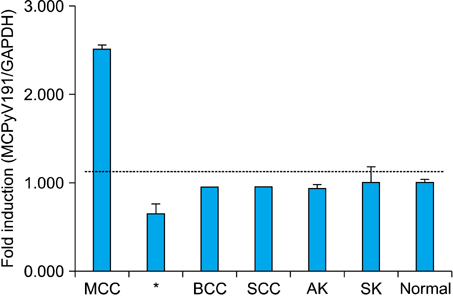
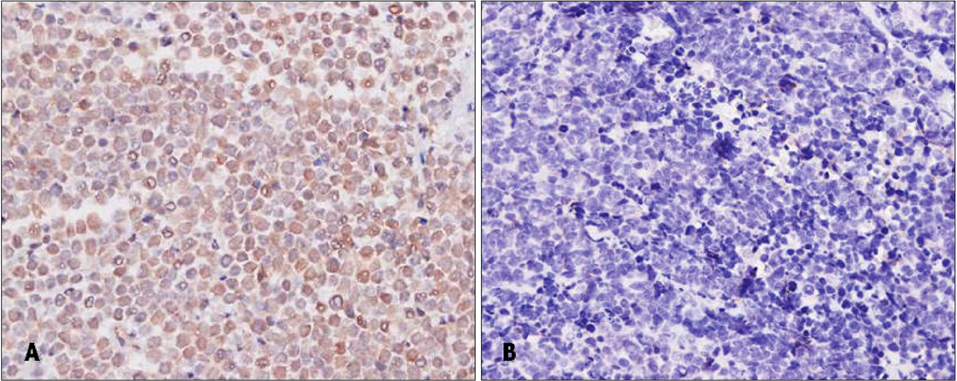
A molecular pathology study was performed on 7 tissue specimens of MCC, 1 tissue specimen of metastatic small cell carcinoma of the lung, and 32 tissue specimens of non-MCC tumors occurring from sun-exposed areas [8 basal cell carcinomas (BCCs), 8 squamous cell carcinomas (SCCs), 8 actinic keratoses (AKs), and 8 seborrheic keratoses (SKs)]. All specimens were analyzed to determine the presence of MCPyV-DNA using both polymerase chain reaction (PCR) and real-time quantitative PCR. Immunohistochemistry with monoclonal antibody of MCPyV large T antigen (CM2B4) was also conducted.
RESULTS
Using both PCR, MCPyV sequences were detected in six of seven MCC tissue specimens (85.7%). Five (71%) of seven MCC tumors were immunoreactive for CM2B4. All five immunoreactive cases were positive for MCPyV. However, there was no association of MCPyV with BCC, SCC, AK, and SK.
CONCLUSION
Our results implicate that MCPyV may contribute to the pathogenesis of primary MCC, not of non-MCC skin tumors in Korea, and the persons with MCPyV infection are unusual in Korea compared to other areas.
MeSH Terms
-
Antigens, Viral, Tumor
Carcinoma, Basal Cell
Carcinoma, Merkel Cell
Carcinoma, Small Cell
Carcinoma, Squamous Cell
Humans
Immunohistochemistry
Keratosis, Actinic
Keratosis, Seborrheic
Korea
Lung
Merkel cell polyomavirus
Pathology, Molecular
Polymerase Chain Reaction
Prevalence
Skin
Skin Neoplasms
Antigens, Viral, Tumor
Figure
Cited by 1 articles
-
A Case of Merkel Cell Carcinoma on the Finger
Jong-Kil Seo, Hyung-Jin Park, Min Kyung Shin, Ki-Heon Jeong
Ann Dermatol. 2019;31(3):357-358. doi: 10.5021/ad.2019.31.3.357.
Reference
-
1. Bichakjian CK, Lowe L, Lao CD, Sandler HM, Bradford CR, Johnson TM, et al. Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer. 2007. 110:1–12.
Article2. Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol. 2009. 27:4021–4026.3. Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971. 1:1253–1257.
Article4. Padgett BL, Walker DL. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973. 127:467–470.
Article5. Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, et al. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007. 3:e64.
Article6. Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, et al. Identification of a third human polyomavirus. J Virol. 2007. 81:4130–4136.
Article7. Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008. 319:1096–1100.
Article8. Kassem A, Schöpflin A, Diaz C, Weyers W, Stickeler E, Werner M, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008. 68:5009–5013.
Article9. Becker JC, Houben R, Ugurel S, Trefzer U, Pföhler C, Schrama D. MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermatol. 2009. 129:248–250.
Article10. Foulongne V, Kluger N, Dereure O, Brieu N, Guillot B, Segondy M. Merkel cell polyomavirus and Merkel cell carcinoma, France. Emerg Infect Dis. 2008. 14:1491–1493.
Article11. Rollison DE, Giuliano AR, Messina JL, Fenske NA, Cherpelis BS, Sondak VK, et al. Case-control study of Merkel cell polyomavirus infection and cutaneous squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2012. 21:74–81.
Article12. Mangana J, Dziunycz P, Kerl K, Dummer R, Cozzio A. Prevalence of Merkel cell polyomavirus among Swiss Merkel cell carcinoma patients. Dermatology. 2010. 221:184–188.
Article13. Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol. 2009. 129:246–248.
Article14. Kassem A, Technau K, Kurz AK, Pantulu D, Löning M, Kayser G, et al. Merkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patients. Int J Cancer. 2009. 125:356–361.
Article15. Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernández-Figueras MT, et al. Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009. 125:1243–1249.
Article16. Andres C, Belloni B, Puchta U, Sander CA, Flaig MJ. Prevalence of MCPyV in Merkel cell carcinoma and non-MCC tumors. J Cutan Pathol. 2010. 37:28–34.
Article17. Holländerová D, Raslová H, Blangy D, Forstová J, Berebbi M. Interference of mouse polyomavirus with the c-myc gene and its product in mouse mammary adenocarcinomas. Int J Oncol. 2003. 23:333–341.
Article18. Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001. 11:15–23.
Article19. Busam KJ, Jungbluth AA, Rekthman N, Coit D, Pulitzer M, Bini J, et al. Merkel cell polyomavirus expression in merkel cell carcinomas and its absence in combined tumors and pulmonary neuroendocrine carcinomas. Am J Surg Pathol. 2009. 33:1378–1385.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Merkel Cell Polyomavirus-Negative Merkel Cell Carcinoma Concurrent with Squamous Cell Carcinoma
- Merkel Cell Carcinoma
- Cytokeratin 20 negative Merkel cell carcinoma consistent with negative Merkel cell polyomavirus
- A Case of Merkel Cell Carcinoma Concurrent with Bowen's Disease
- Cervical Spinal Metastasis of Merkel Cell Carcinoma