Endocrinol Metab.
2016 Mar;31(1):7-16. 10.3803/EnM.2016.31.1.7.
Understanding Metabolomics in Biomedical Research
- Affiliations
-
- 1Biomedical Research Center, Department of Convergence Medicine, Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. yoohyunju@amc.seoul.kr
- 2Division of Liver Transplantation and Hepatobiliary Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2169686
- DOI: http://doi.org/10.3803/EnM.2016.31.1.7
Abstract
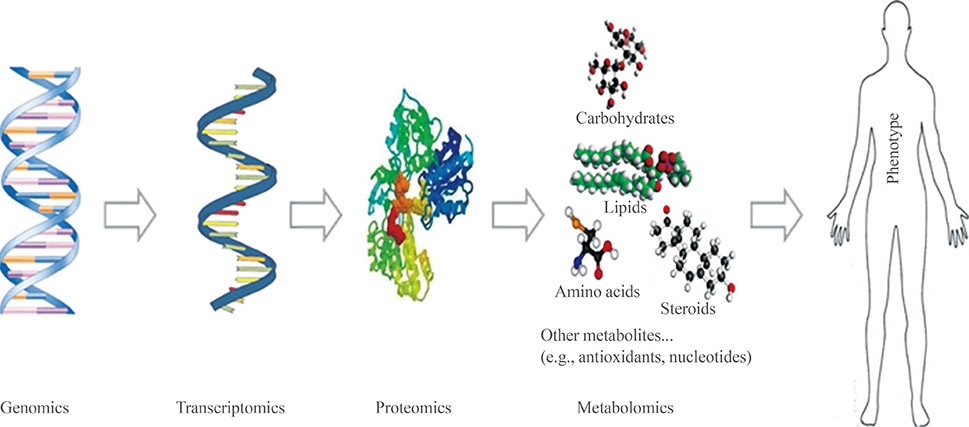
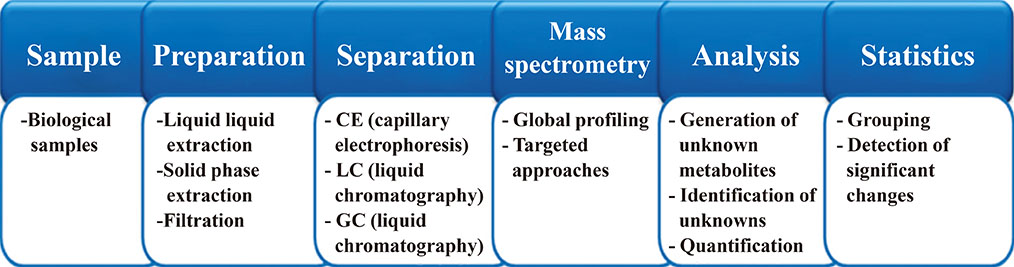
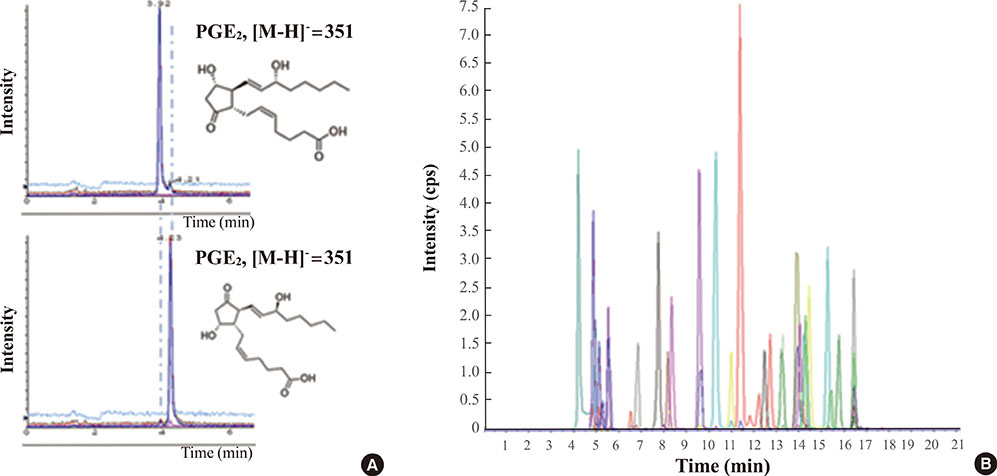
- The term "omics" refers to any type of specific study that provides collective information on a biological system. Representative omics includes genomics, proteomics, and metabolomics, and new omics is constantly being added, such as lipidomics or glycomics. Each omics technique is crucial to the understanding of various biological systems and complements the information provided by the other approaches. The main strengths of metabolomics are that metabolites are closely related to the phenotypes of living organisms and provide information on biochemical activities by reflecting the substrates and products of cellular metabolism. The transcriptome does not always correlate with the proteome, and the translated proteome might not be functionally active. Therefore, their changes do not always result in phenotypic alterations. Unlike the genome or proteome, the metabolome is often called the molecular phenotype of living organisms and is easily translated into biological conditions and disease states. Here, we review the general strategies of mass spectrometry-based metabolomics. Targeted metabolome or lipidome analysis is discussed, as well as nontargeted approaches, with a brief explanation of the advantages and disadvantages of each platform. Biomedical applications that use mass spectrometry-based metabolomics are briefly introduced.
MeSH Terms
Figure
Cited by 1 articles
-
Effect of Pioglitazone on Perihematomal Edema in Intracerebral Hemorrhage Mouse Model by Regulating NLRP3 Expression and Energy Metabolism
Hoon Kim, Jung Eun Lee, Hyun Ju Yoo, Jae Hoon Sung, Seung Ho Yang
J Korean Neurosurg Soc. 2020;63(6):689-697. doi: 10.3340/jkns.2020.0056.
Reference
-
1. Fernie AR, Trethewey RN, Krotzky AJ, Willmitzer L. Metabolite profiling: from diagnostics to systems biology. Nat Rev Mol Cell Biol. 2004; 5:763–769.2. Yoo HJ, Liu H, Hakansson K. Infrared multiphoton dissociation and electron-induced dissociation as alternative MS/MS strategies for metabolite identification. Anal Chem. 2007; 79:7858–7866.3. Villas-Boas SG, Mas S, Akesson M, Smedsgaard J, Nielsen J. Mass spectrometry in metabolome analysis. Mass Spectrom Rev. 2005; 24:613–646.4. Want EJ, Cravatt BF, Siuzdak G. The expanding role of mass spectrometry in metabolite profiling and characterization. Chembiochem. 2005; 6:1941–1951.5. Brown SC, Kruppa G, Dasseux JL. Metabolomics applications of FT-ICR mass spectrometry. Mass Spectrom Rev. 2005; 24:223–231.6. Boskey AL, Mendelsohn R. Infrared spectroscopic characterization of mineralized tissues. Vib Spectrosc. 2005; 38:107–114.7. Defernez M, Colquhoun IJ. Factors affecting the robustness of metabolite fingerprinting using 1H NMR spectra. Phytochemistry. 2003; 62:1009–1017.8. Dunn WB, Ellis DI. Metabolomics: current analytical platforms and methodologies. Trends Analyt Chem. 2005; 24:285–294.9. Deleris G, Petibois C. Applications of FT-IR spectrometry to plasma contents analysis and monitoring. Vib Spectrosc. 2003; 32:129–136.10. Metz TO, Zhang Q, Page JS, Shen Y, Callister SJ, Jacobs JM, et al. The future of liquid chromatography-mass spectrometry (LC-MS) in metabolic profiling and metabolomic studies for biomarker discovery. Biomark Med. 2007; 1:159–185.11. Bino RJ, Hall RD, Fiehn O, Kopka J, Saito K, Draper J, et al. Potential of metabolomics as a functional genomics tool. Trends Plant Sci. 2004; 9:418–425.12. Smith CA, O'Maille G, Want EJ, Qin C, Trauger SA, Brandon TR, et al. METLIN: a metabolite mass spectral database. Ther Drug Monit. 2005; 27:747–751.13. Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, et al. HMDB 3.0: the Human Metabolome Database in 2013. Nucleic Acids Res. 2013; 41(Database issue):D801–D807.14. Edwards JL, Chisolm CN, Shackman JG, Kennedy RT. Negative mode sheathless capillary electrophoresis electrospray ionization-mass spectrometry for metabolite analysis of prokaryotes. J Chromatogr A. 2006; 1106:80–88.15. Buchholz A, Takors R, Wandrey C. Quantification of intracellular metabolites in Escherichia coli K12 using liquid chromatographic-electrospray ionization tandem mass spectrometric techniques. Anal Biochem. 2001; 295:129–137.16. Byrd GD, Ogden MW. Liquid chromatographic/tandem mass spectrometric method for the determination of the tobacco-specific nitrosamine metabolite NNAL in smokers' urine. J Mass Spectrom. 2003; 38:98–107.17. Triolo A, Altamura M, Dimoulas T, Guidi A, Lecci A, Tramontana M. In vivo metabolite detection and identification in drug discovery via LC-MS/MS with data-dependent scanning and postacquisition data mining. J Mass Spectrom. 2005; 40:1572–1582.18. Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomics profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One. 2010; 5:e15234.19. Want EJ, Masson P, Michopoulos F, Wilson ID, Theodoridis G, Plumb RS, et al. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat Protoc. 2013; 8:17–32.20. Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. 2011; 6:1060–1083.21. Osborn MP, Park Y, Parks MB, Burgess LG, Uppal K, Lee K, et al. Metabolome-wide association study of neovascular age-related macular degeneration. PLoS One. 2013; 8:e72737.22. Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, et al. The human urine metabolome. PLoS One. 2013; 8:e73076.23. Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, et al. The human serum metabolome. PLoS One. 2011; 6:e16957.24. Ramautar R, Somsen GW, de Jong GJ. CE-MS in metabolomics. Electrophoresis. 2009; 30:276–291.25. Benton HP, Wong DM, Trauger SA, Siuzdak G. XCMS2: processing tandem mass spectrometry data for metabolite identification and structural characterization. Anal Chem. 2008; 80:6382–6389.26. Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G. XCMS Online: a web-based platform to process untargeted metabolomic data. Anal Chem. 2012; 84:5035–5039.27. Katajamaa M, Miettinen J, Oresic M. MZmine: toolbox for processing and visualization of mass spectrometry based molecular profile data. Bioinformatics. 2006; 22:634–636.28. Krieger CJ, Zhang P, Mueller LA, Wang A, Paley S, Arnaud M, et al. MetaCyc: a multiorganism database of metabolic pathways and enzymes. Nucleic Acids Res. 2004; 32(Database issue):D438–D442.29. Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000; 28:27–30.30. Caspi R, Altman T, Billington R, Dreher K, Foerster H, Fulcher CA, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res. 2014; 42(Database issue):D459–D471.31. Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. 2012; 7:872–881.32. Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010; 51:3299–3305.33. Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med. 2011; 365:1812–1823.34. Kim SJ, Kim N, Koh EH, Yoo HJ. Identification of ethanolamine plasmalogens from complex lipid mixtures by MS/MS and Ag adduction. Anal Sci. 2012; 28:1207–1212.35. Kim SJ, Back SH, Koh JM, Yoo HJ. Quantitative determination of major platelet activating factors from human plasma. Anal Bioanal Chem. 2014; 406:3111–3118.36. Huda-Faujan N, Abdulamir AS, Fatimah AB, Anas OM, Shuhaimi M, Yazid AM, et al. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem J. 2010; 4:53–58.37. Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009; 81:8085–8093.38. Tietz NW. Clinical guide to laboratory tests. Philadelphia: WB Saunders;1995.39. Wishart DS. Metabolomics: applications to food science and nutrition research. Trends Food Sci Technol. 2008; 19:482–493.40. Wang C, Kong H, Guan Y, Yang J, Gu J, Yang S, et al. Plasma phospholipid metabolic profiling and biomarkers of type 2 diabetes mellitus based on high-performance liquid chromatography/electrospray mass spectrometry and multivariate statistical analysis. Anal Chem. 2005; 77:4108–4116.41. Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, Nair KS. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS One. 2010; 5:e10538.42. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009; 9:311–326.43. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011; 17:448–453.44. Zhao X, Fritsche J, Wang J, Chen J, Rittig K, Schmitt-Kopplin P, et al. Metabonomic fingerprints of fasting plasma and spot urine reveal human pre-diabetic metabolic traits. Metabolomics. 2010; 6:362–374.45. Maeda S. Pathology of experimental radiation pancarditis. II. Correlation between ultrastructural changes of the myocardial mitochondria and succinic dehydrogenase activity in rabbit heart receiving a single dose of X-ray irradiation. Acta Pathol Jpn. 1982; 32:199–218.46. Guilbault C, Wojewodka G, Saeed Z, Hajduch M, Matouk E, De Sanctis JB, et al. Cystic fibrosis fatty acid imbalance is linked to ceramide deficiency and corrected by fenretinide. Am J Respir Cell Mol Biol. 2009; 41:100–106.47. Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008; 14:382–391.48. Li C, Zheng S, You H, Liu X, Lin M, Yang L, et al. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. J Hepatol. 2011; 54:1205–1213.49. Moustafa T, Fickert P, Magnes C, Guelly C, Thueringer A, Frank S, et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology. 2012; 142:140–151.e12.50. Aubin MC, Lajoie C, Clement R, Gosselin H, Calderone A, Perrault LP. Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: therapeutic potential of resveratrol. J Pharmacol Exp Ther. 2008; 325:961–968.51. Levick SP, Loch DC, Taylor SM, Janicki JS. Arachidonic acid metabolism as a potential mediator of cardiac fibrosis associated with inflammation. J Immunol. 2007; 178:641–646.52. Castelino FV. Lipids and eicosanoids in fibrosis: emerging targets for therapy. Curr Opin Rheumatol. 2012; 24:649–655.53. Cha JH, Bae SH, Kim HL, Park NR, Choi ES, Jung ES, et al. Branched-chain amino acids ameliorate fibrosis and suppress tumor growth in a rat model of hepatocellular carcinoma with liver cirrhosis. PLoS One. 2013; 8:e77899.54. Warburg O. On the origin of cancer cells. Science. 1956; 123:309–314.55. Kim YS, Maruvada P. Frontiers in metabolomics for cancer research: Proceedings of a National Cancer Institute workshop. Metabolomics. 2008; 4:105–113.56. Kim K, Aronov P, Zakharkin SO, Anderson D, Perroud B, Thompson IM, et al. Urine metabolomics analysis for kidney cancer detection and biomarker discovery. Mol Cell Proteomics. 2009; 8:558–570.57. Griffin JL, Shockcor JP. Metabolic profiles of cancer cells. Nat Rev Cancer. 2004; 4:551–561.58. Kim S, Rhee JK, Yoo HJ, Lee HJ, Lee EJ, Lee JW, et al. Bioinformatic and metabolomic analysis reveals miR-155 regulates thiamine level in breast cancer. Cancer Lett. 2015; 357:488–497.59. Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010; 10:181–193.60. Hilvo M, Denkert C, Lehtinen L, Muller B, Brockmoller S, Seppanen-Laakso T, et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011; 71:3236–3245.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metabolomics 2014 Tenth annual international conference of the metabolomics society
- Metabolomics Goes to Work for You
- Metabolomics Research in Kidney Transplantation
- Complex influences of gut microbiome metabolism on various drug responses
- Recent advances in nonalcoholic fatty liver disease metabolomics