J Bacteriol Virol.
2016 Mar;46(1):27-35. 10.4167/jbv.2016.46.1.27.
Effect of Methylene Blue-mediated Photodynamic Therapy on Wild-type and Ciprofloxacin-resistant Mycobacterium smegmatis
- Affiliations
-
- 1Department of Microbiology, Kosin University College of Medicine, Busan, Korea. indalp103@gmail.com
- 2Department of Biochemistry, Kosin University College of Medicine, Busan, Korea.
- 3Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea. oaks70@daum.net
- KMID: 2168740
- DOI: http://doi.org/10.4167/jbv.2016.46.1.27
Abstract
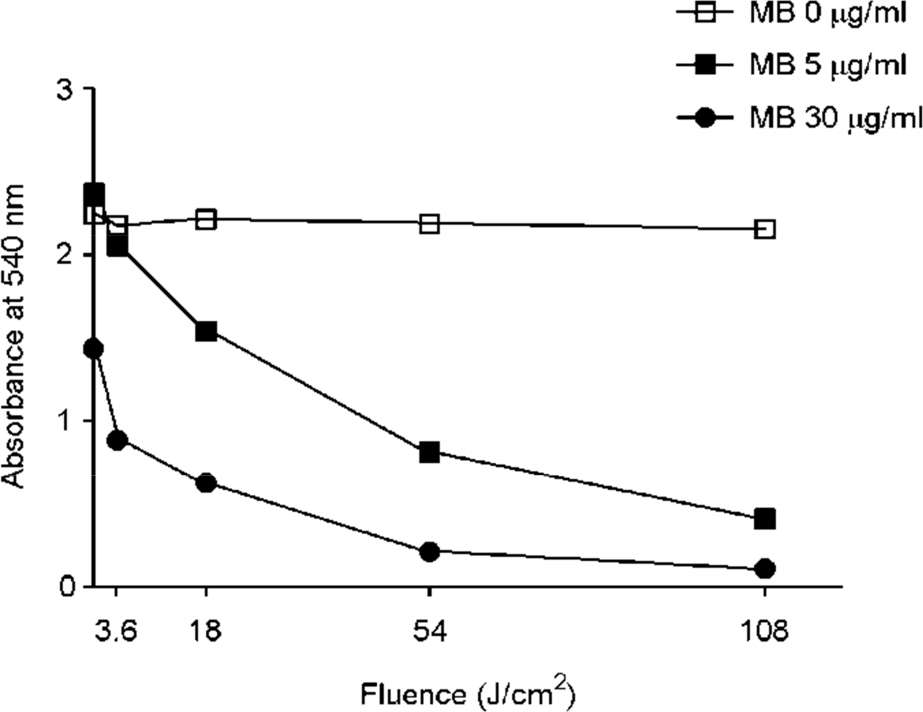
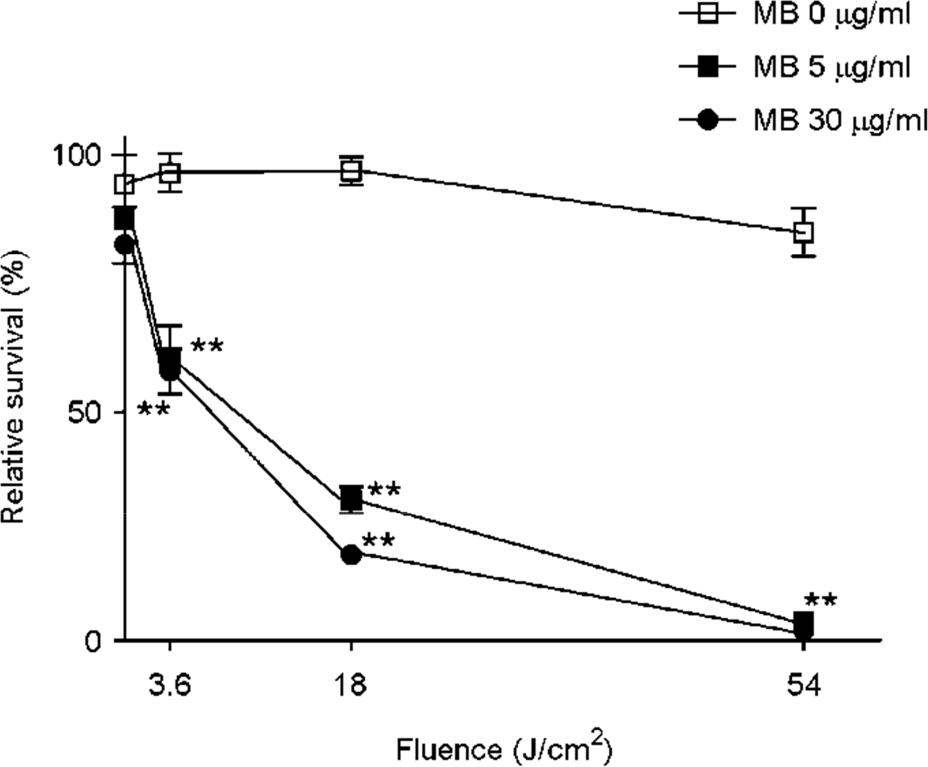
- Tuberculosis (TB) patients are normally treated with a combination of antibiotics. However, with improper or incomplete treatment of antibiotics, the disease may progress to multidrug-resistant TB (MDR-TB). The treatment of MDR-TB is very costly and inefficient. Therefore, there is a great demand of new therapeutic approaches for MDR-TB such as photodynamic therapy. In this study, we tried to optimize the conditions for photodynamic inactivation of TB using methylene blue as a photosensitizer. Different combinations of methylene blue concentrations and light doses were tested for their photodynamic effects to A549 cells or Mycobacterium smegmatis (M. smegmatis). We also tested the effect of photodynamic therapy on ciprofloxacin-resistant M. smegmatis. Methylene blue treatment alone did not affect the survival rates of A549 cells or bacteria up to 5 µg/ml. When the A549 and M. smegmatis cells treated with methylene blue were irradiated with laser light (wavelength, 630 nm), photodynamic inactivation of cells was increased in methylene blue concentration- and light dose-dependent manners. Interestingly, the ciprofloxacin-resistant M. smegmatis exhibited higher level of susceptibility to methylene blue-mediated photodynamic inactivation. This study suggests that photodynamic therapy at 3.6 J/cm2 in the presence of 5 µg/ml methylene blue may be an appropriate range for therapy due to the high bactericidal activity against high level of ciprofloxacin-resistant M. smegmatis and the low damaging effect to mammalian cells. This study demonstrates that photodynamic therapy could be a potential alternative for MDR-TB treatment.
MeSH Terms
Figure
Reference
-
1). Dougherty TJ. Photodynamic therapy. Photochem Photobiol. 1993; 58:895–900.
Article2). Allison RR, Downie GH, Cuenca R, Hu X, Childs CJH, Sibata CH. Photosensitizers in clinical PDT. Photodiag Photodynamic. 2004; 1:27–42.
Article3). Brancaleon L, Moseley H. Laser and non-laser light sources for photodynamic therapy. Lasers Med Sci. 2002; 17:173–86.
Article4). Daniela V, Asheesh G, Liyi H, Giacomo L, Pinar A, Andrea R, et al. Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrob Agents Chemother. 2015; 59:5203–12.5). Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003; 3:380–7.
Article6). Feese E, Ghiladi RA. Highly efficient in vitro photodynamic inactivation of Mycobacterium smegmatis. J Antimicrob Chemother. 2009; 64:782–785.7). Gudgin DEF, Goyan RL, Pottier RH. New directions in photodynamic therapy. Cell Mol Bio. 2002; 48:939–54.8). Janouskova O, Rakusan J, Karaskova M, Holada K. Photodynamic inactivation of prions by disulfonated hydroxyaluminium phthalocyanine. J Gen Virol. 2012; 93:2512–7.
Article9). Song D, Lindoso JA, Oyafuso LK, Kanashiro EH, Cardoso JL, Uchoa AF, et al. Photodynamic therapy using methylene blue to treat cutaneous leishmaniasis. Photomed Laser Surg. 2011; 29:711–5.
Article10). Sperandio FF, Huang Y, Hamblin MR. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat Antiinfect Drug Discov. 2013; 8:108–20.
Article11). Usuda J, Kato H, Okunaka T, Furukawa K, Tsutsui H, Yamada K, et al. Photodynamic therapy (PDT) for lung cancers. J Thorac Oncol. 2006; 1:489–93.
Article12). Wong TW, Huang HJ, Wang YF, Lee YP, Huang CC, Yu CK. Methylene blue mediated photodynamic inactivation as a novel disinfectant of enterovirus 71. J Antimicrob Chemother. 2010; 65:2176–82.13). Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, et al. Treatment outcomes and survival based on drug resistance patterns in multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2011; 182:113–9.
Article14). Kim HJ. Current status of tuberculosis in Korea. Korean J Med. 2012; 82:257–62.
Article15). Park MM, Davis AL, Schluger NW, Cohen H, Rom WN. Outcome of MDR-TB patients, 1983–1993. Prolonged survival with appropriate therapy. Am J Respir Crit Care Med. 1996; 153:317–24.
Article16). Devasia R, Blackman A, Eden S, Li H, Maruri F, Shintani A, et al. High proportion of fluoroquinoloneresistant Mycobacterium tuberculosis isolates with novel gyrase polymorphisms and a gyr A region associated with fluoroquinolone susceptibility. J Clin Microbiol. 2012; 50:1390–6.17). Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998; 42:2066–9.18). Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998; 393:537–44.19). Groll AV, Martin A, Jureen P, Hoffner S, Vandamme P, Portaels F, et al. Fluoroquinolone resistance in Mycobacterium tuberculosis and mutations in gyr A and gyr B. Antimicrob Agents Chemother. 2009; 53:4498–500.20). Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyr A gene of Escherichia coli. Antimicrob Agents Chemother. 1990; 34:1271–2.21). Ferrero L, Cameron B, Crouzet J. Analysis of gyr A and grl A mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995; 39:1554–8.22). Sturmey RG, Wild CP, Hardie LJ. Removal of red light minimizes methylene blue-stimulated DNA damage in oesophageal cells: implications for chromoendoscopy. Mutagenesis. 2009; 24:253–8.
Article23). Davies J, Burke D, Olliver JR, Hardie LJ, Wild CP, Routledge MN. Methylene blue but not indigo carmine causes DNA damage to colonocytes in vitro and in vivo at concentrations used in clinical chromoendoscopy. Gut. 2007; 56:155–6.24). Shih MH, Huang FC. Effects of photodynamic therapy on rapidly growing nontuberculous mycobacteria keratitis. Invest Ophthalmol Vis Sci. 2011; 52:223–9.
Article25). Sirgel FA, Warren RM, Streicher EM, Victor TC, Helden PD, Bööttger EC. gyr A mutations and phenotypic susceptibility levels to ofloxacin and moxifloxacin in clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother. 2012; 67:1088–93.26). Yokoyama K, Kim H, Mukai T, Matsuoka M, Nakajima C, Suzuki Y. Amino acid substitutions at position 95 in GyrA can add fluoroquinolone resistance to Mycobacterium leprae. Antimicrob Agents Chemother. 2012; 56:697–702.27). Sung N, Back S, Jung J, Kim K, Kim J, Lee JH, et al. Inactivation of multidrug resistant (MDR)-and extensively drug resistant (XDR)-Mycobacterium tuberculosis by photodynamic therapy. Photodiagn Photodyn Ther. 2013; 10:694–702.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Photodynamic Therapy Enhanced by Methylene Blue on Drug-resistant Mycobacterium smegmatis
- Microgel-Encapsulated Methylene Blue for the Treatment of Breast Cancer Cells by Photodynamic Therapy
- Effects of methylene blue or evan's blue on mouse embryo development, human sperm motility, serum E2 level and components of human intra tubal fluid
- Interactions between ciprofloxacin and other antituberculous drugs in the growth inhibition of mycobacterium tuberculosis
- The Effect after Intra-dermal Methylene Blue, Hydrocortisone, Lidocaine Injection Therapy for Intractable, Idiopathic Pruritus Ani