J Bacteriol Virol.
2012 Dec;42(4):294-304. 10.4167/jbv.2012.42.4.294.
Coordinate Regulation of Vibrio vulnificus Heme Receptor HupA Expression by Cyclic AMP-receptor Protein and Ferric Uptake Regulator
- Affiliations
-
- 1Department of Emergence Medicine, Chosun University Medical School, Gwangju, Korea.
- 2Research Center for Resistant Cells, Chosun University Medical School, Gwangju, Korea. shsin@chosun.ac.kr
- 3Department of Microbiology, Chosun University Medical School, Gwangju, Korea.
- KMID: 2168664
- DOI: http://doi.org/10.4167/jbv.2012.42.4.294
Abstract
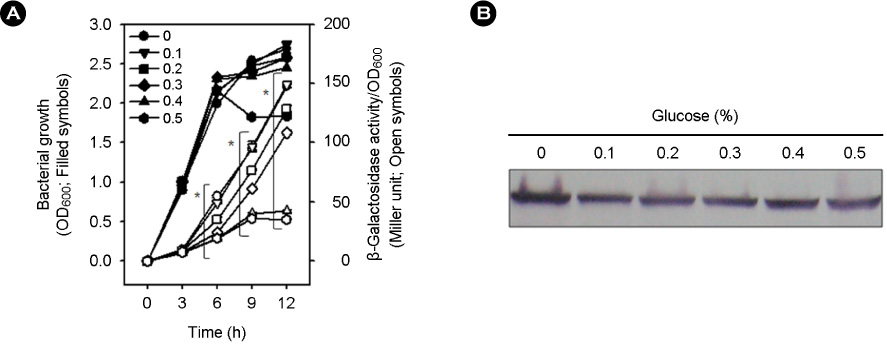
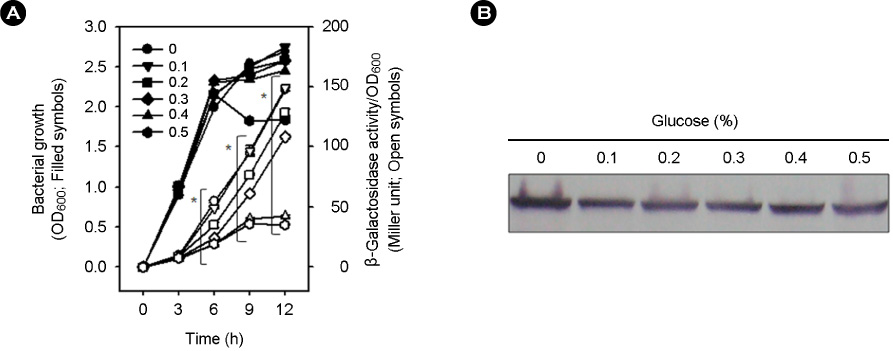
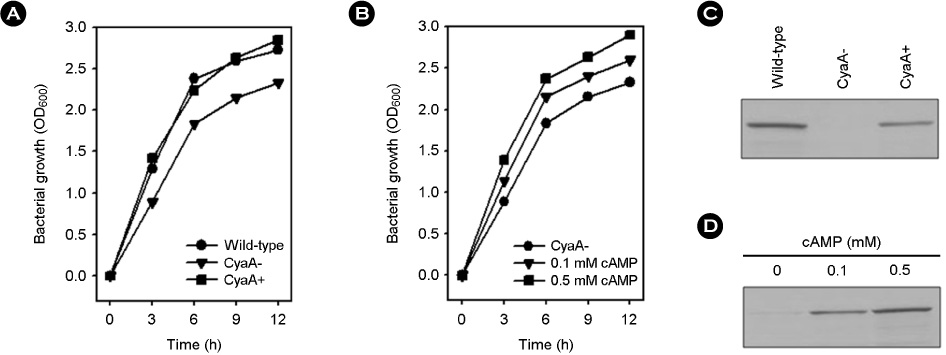
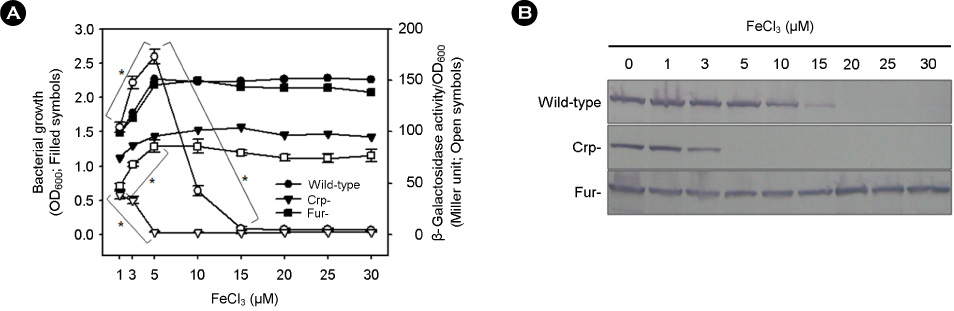
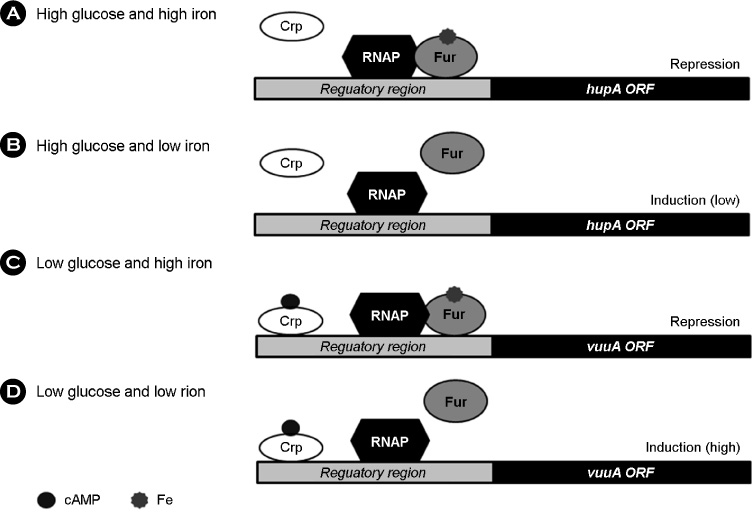
- Vibrio vulnificus causes rapid progressing fulminant infections in susceptible individuals, especially those with elevated serum iron levels. This ferrophilic bacterium can directly acquire iron from heme-containing proteins, such as, hemoglobin, via its heme receptor protein HupA. This study was undertaken to determine the roles of cyclic AMP-receptor protein (Crp) as an activator and of ferric uptake regulator (Fur) as a repressor in regulating hupA expression at various iron and glucose concentrations. Under severely iron-deficient conditions, hupA expression in the absence of Crp was induced albeit at low levels and repressed by the addition of iron. In contrast, hupA expression in the presence of Crp was increased by the addition of iron. Under moderately iron-deficient and iron-sufficient conditions, iron addition repressed hupA expression in the presence of Fur, but not in the absence of Fur. Glucose addition repressed hupA expression in the presence of Fur but not in the absence of Fur. Furthermore, a mutation in cyaA encoding adenylate cyclase required for cAMP synthesis hupA expression, and this repression was prevented by the exogenous addition of cAMP. These results indicate that hupA expression is under the coordinate control of cAMP or Crp, which responds to glucose availability, and of Fur, which responds to iron availability, and that Crp is not essential for the constitutional expression of hupA, but is required for the optimal expression of hupA, whereas Fur is essential for the prevention of hupA over-expression.
Keyword
MeSH Terms
Figure
Reference
-
1. Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009. 77:1723–1733.2. Wright AC, Simpson LM, Oliver JD. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981. 34:503–507.
Article3. Stelma GN Jr, Reyes AL, Peeler JT, Johnson CH, Spaulding PL. Virulence characteristics of clinical and environmental isolates of Vibrio vulnificus. Appl Environ Microbiol. 1992. 58:2776–2782.
Article4. Bullen JJ, Spalding PB, Ward CG, Gutteridge JM. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991. 151:1606–1609.
Article5. Weinberg ED. Microbial pathogens with impaired ability to acquire host iron. BioMetals. 2000. 13:85–89.6. Kim CM, Park RY, Choi MH, Sun HY, Shin SH. Ferrophilic characteristics of Vibrio vulnificus and potential usefulness of iron chelation therapy. J Infect Dis. 2007. 195:90–98.
Article7. Helms SD, Oliver JD, Travis JC. Role of heme compounds and haptoglobin in Vibrio vulnificus pathogenicity. Infect Immun. 1984. 45:345–349.
Article8. Gray LD, Kreger AS. Mouse skin damage caused by cytolysin from Vibrio vulnificus and by V. vulnificus infection. J Infect Dis. 1987. 155:236–241.
Article9. Kim YR, Lee SE, Kook H, Yeom JA, Na HS, Kim SY, et al. Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell Microbiol. 2008. 10:848–862.
Article10. Oh MH, Lee SM, Lee DH, Choi SH. Regulation of the Vibrio vulnificus hupA gene by temperature alteration and cyclic AMP receptor protein and evaluation of its role in virulence. Infect Immun. 2009. 77:1208–1215.
Article11. Litwin CM, Calderwood SB. Cloning and genetic analysis of the Vibrio vulnificus fur gene and construction of a fur mutant by in vivo marker exchange. J Bacteriol. 1993. 175:706–715.
Article12. Litwin CM, Byrne BL. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect Immun. 1998. 66:3134–3141.
Article13. Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr Opin Microbiol. 2008. 11:87–93.
Article14. Martínez-Antonio A, Collado-Vides J. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr Opin Microbiol. 2003. 6:482–489.
Article15. Reddy GP, Hayat U, Abeygunawardana C, Fox C, Wright AC, Maneval DR Jr, et al. Purification and determination of the structure of capsular polysaccharide of Vibrio vulnificus M06-24. J Bacteriol. 1992. 174:2620–2630.
Article16. Choi MH, Sun HY, Park RY, Kim CM, Bai YH, Kim YR, et al. Effect of the crp mutation on the utilization of transferrin-bound iron by Vibrio vulnificus. FEMS Microbiol Lett. 2006. 257:285–292.
Article17. Kim CM, Park RY, Park JH, Sun HY, Bai YH, Ryu PY, et al. Vibrio vulnificus vulnibactin, but not metalloprotease VvpE, is essentially required for iron-uptake from human holotransferrin. Biol Pharm Bull. 2006. 29:911–918.
Article18. Kim SY, Lee SE, Kim YR, Kim CM, Ryu PY, Choy HE, et al. Regulation of Vibrio vulnificus virulence by the LuxS quorum-sensing system. Mol Microbiol. 2003. 48:1647–1664.
Article19. Kim CM, Shin SH. Regulation of the Vibrio vulnificus vvpE expression by cyclic AMP-receptor protein and quorum-sensing regulator SmcR. Microb Pathog. 2010. 49:348–353.
Article20. Kim CM, Chung YY, Shin SH. Iron differentially regulates gene expression and extracellular secretion of Vibrio vulnificus cytolysin-hemolysin. J Infect Dis. 2009. 200:582–589.
Article21. Kim CM, Shin SH. Modulation of iron-uptake systems by a mutation of luxS encoding an autoinducer-2 sysnthase in Vibrio vulnificus. Biol Pharm Bull. 2011. 34:632–637.
Article22. Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: Osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988. 170:2575–2583.
Article23. McGee K, Hörstedt P, Milton DL. Identification and characterization of additional flagellin genes from Vibrio anguillarum. J Bacteriol. 1996. 178:5188–5198.
Article24. Ditta G, Stanfield S, Corbin D, Helinski DR. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980. 27:7347–7351.25. Simpson LM, Oliver JD. Siderophore production by Vibrio vulnificus. Infect Immun. 1983. 41:644–649.26. Miller JH. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. 1992. New York: Cold Spring Harbor Laboratory Press;72–74.27. Kim CM, Kim SJ, Shin SH. Cyclic AMP-receptor protein activates aerobactin receptor IutA expression in Vibrio vulnificus. J Microbiol. 2012. 50:320–325.
Article28. Choi HK, Park NY, Kim DI, Chung HJ, Ryu S, Choi SH. Promoter analysis and regulatory characteristics of vvhBA encoding cytolytic hemolysin of Vibrio vulnificus. J Biol Chem. 2002. 277:47292–47299.
Article29. Kim HS, Shin SH, Park HR, Lee SE, Kim CM, Kim SY, et al. Effect of salinity, temperature, and glucose on the production of Vibrio vulnificus hemolysin. J Bacteriol Virol. 2002. 32:355–365.30. Webster AC, Litwin CM. Cloning and characterization of vuuA, a gene encoding Vibrio vulnificus ferric vulnibactin receptor. Infect Immun. 2000. 68:526–534.
Article31. Kim CM, Park YJ, Shin SH. A widespread deferoxamine-mediated iron-uptake system in Vibrio vulnificus. J Infect Dis. 2007. 196:1537–1545.
Article32. Tanabe T, Naka A, Aso H, Nakao H, Narimatsu S, Inoue Y, et al. A novel aerobactin utilization cluster in Vibrio vulnificus with a gene involved in the transcription regulation of the iutA homologue. Microbiol Immunol. 2005. 49:823–834.
Article33. Shin SH, Chung SS, Rhee JH. Phenolate siderophore stimulates growth of Vibrio vulnificus. J Bacteriol Virol. 2001. 31:325–331.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cyclic AMP and Cyclic AMP-Receptor Protein are Required for Optimal Capsular Polysaccharide Expression
- Multiple High-affinity Iron-uptake Systems of Vibrio vulnificus
- Vibrio vulnificus Hemolysin Is Easily Inactivated in Spite of Being Produced at High Levels in Cirrhotic Ascites by a fur Mutation
- Effect of CRP (cAMP Receptor Protein) of Escherichia coli on Transcription Initiation at lacUV5 Promoter
- Effect of intracelluar cyclic AMP on EGF receptor binding in human gastric adenocarcinoma cells