Cancer Res Treat.
2014 Jan;46(1):19-26.
A Randomized Double-Blind, Double-Dummy, Multicenter Trial of Azasetron versus Ondansetron to Evaluate Efficacy and Safety in the Prevention of Delayed Nausea and Vomiting Induced by Chemotherapy
- Affiliations
-
- 1Department of Internal Medicine, St. Vincent's Hospital, The Catholic University of Korea College of Medicine, Suwon, Korea. miongsok@catholic.ac.kr
- 2Department of Internal Medicine, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea.
- 3Department of Internal Medicine, Seoul Veterans Hospital, Seoul, Korea.
- 4Department of Internal Medicine, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
- 5Department of Internal Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 6Department of Internal Medicine, Inje University Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 7Department of Internal Medicine, Korea University Anam Hospital, Korea University School of Medicine, Seoul, Korea.
- 8Department of Internal Medicine, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea.
- 9Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 11Department of Internal Medicine, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, Korea.
- 12Department of Internal Medicine, Soonchunhyang University Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
This study was conducted to evaluate the efficacy and safety of azasetron compared to ondansetron in the prevention of delayed chemotherapy-induced nausea and vomiting.
MATERIALS AND METHODS
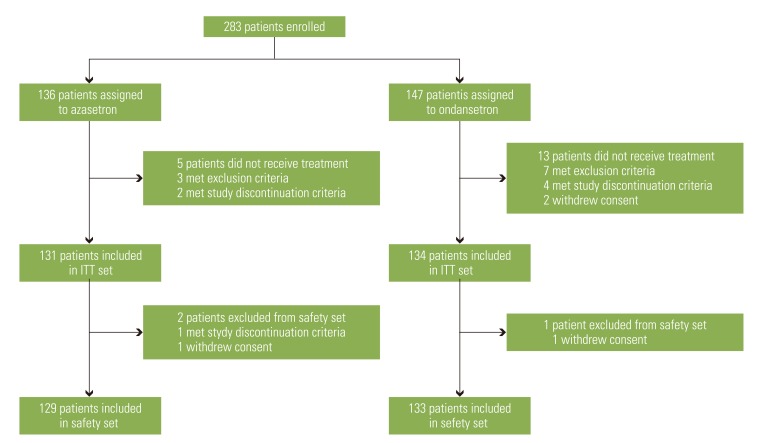
This study was a multi-center, prospective, randomized, double-dummy, double-blind and parallel-group trial involving 12 institutions in Korea between May 2005 and December 2005. A total of 265 patients with moderately and highly emetogenic chemotherapy were included and randomly assigned to either the azasetron or ondansetron group. All patients received azasetron (10 mg intravenously) and dexamethasone (20 mg intravenously) on day 1 and dexamethasone (4 mg orally every 12 hours) on days 2-4. The azasetron group received azasetron (10 mg orally) with placebo of ondansetron (orally every 12 hours), and the ondansetron group received ondansetron (8 mg orally every 12 hours) with placebo of azasetron (orally) on days 2-6.
RESULTS
Over days 2-6, the effective ratio of complete response in the azasetron and ondansetron groups was 45% and 54.5%, respectively (95% confidence interval, -21.4 to 2.5%). Thus, the non-inferiority of azasetron compared with ondansetron in delayed chemotherapy-induced nausea and vomiting was not proven in the present study. All treatments were well tolerated and no unexpected drug-related adverse events were reported. The most common adverse events related to the treatment were constipation and hiccups, and there were no differences in the overall incidence of adverse events.
CONCLUSION
In the present study, azasetron showed inferiority in the control of delayed chemotherapy-induced nausea and vomiting compared with ondansetron whereas safety profiles were similar between the two groups.
Keyword
MeSH Terms
Figure
Reference
-
1. Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, et al. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer. 2005; 13:219–227. PMID: 15538640.
Article2. Cheirsilpa A, Sinthusake T, Songsakkaesorn A, Visawaprasit S, Chulaka K, Changkuingdee N. Comparison of ramosetron and granisetron for the prevention of acute and delayed emesis in cisplatin-based chemotherapy: a randomized controlled trial. Jpn J Clin Oncol. 2005; 35:695–699. PMID: 16319109.
Article3. Yun MJ, Kim YH, Kim AR. Comparison of azasetron and ondansetron for preventing postoperative nausea and vomiting in patients undergoing gynecological laparoscopic surgery. Yonsei Med J. 2010; 51:88–92. PMID: 20046519.
Article4. Navari RM. Pathogenesis-based treatment of chemotherapy-induced nausea and vomiting: two new agents. J Support Oncol. 2003; 1:89–103. PMID: 15352652.5. Sakamori M, Takehara S, Setoguchi M. High affinity binding of Y-25130 for serotonin 3 receptor. Nihon Yakurigaku Zasshi. 1992; 100:137–142. PMID: 1330854.
Article6. Tsukagoshi S. Pharmacokinetics of azasetron (Serotone), a selective 5-HT3 receptor antagonist. Gan To Kagaku Ryoho. 1999; 26:1001–1008. PMID: 10396331.7. Hayakawa T, Sato M, Konaka M, Makino A, Hirohata T, Totsu S, et al. Comparison of ramosetron and azasetron for prevention of acute and delayed cisplatin-induced emesis in lung cancer patients. Gan To Kagaku Ryoho. 2006; 33:633–638. PMID: 16685162.8. Kimura E, Niimi S, Watanabe A, Tanaka T. Clinical effect of two azasetron treatment methods against nausea and vomiting induced by anticancer drugs including CDDP. Gan To Kagaku Ryoho. 1997; 24:855–859. PMID: 9170525.9. Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997; 15:103–109. PMID: 8996130.
Article10. Ishiwata K, Ishii K, Ishii S, Senda M. Synthesis of 5-HT3 receptor antagonists, [11C] Y-25130 and [11C] YM060. Appl Radiat Isot. 1995; 46:907–910. PMID: 7581293.11. Yang SH, Lee MG. Dose-independent pharmacokinetics of ondansetron in rats: contribution of hepatic and intestinal first-pass effects to low bioavailability. Biopharm Drug Dispos. 2008; 29:414–426. PMID: 18697186.
Article12. Simpson KH, Murphy P, Colthup PV, Whelan P. Concentration of ondansetron in cerebrospinal fluid following oral dosing in volunteers. Psychopharmacology (Berl). 1992; 109:497–498. PMID: 1365869.
Article13. Rudd JA, Naylor RJ. Effects of 5-HT3 receptor antagonists on models of acute and delayed emesis induced by cisplatin in the ferret. Neuropharmacology. 1994; 33:1607–1608. PMID: 7760983.14. Geling O, Eichler HG. Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol. 2005; 23:1289–1294. PMID: 15718327.
Article15. Haga K, Inaba K, Shoji H, Morimoto Y, Fukuda T, Setoguchi M. The effects of orally administered Y-25130, a selective serotonin3-receptor antagonist, on chemotherapeutic agent-induced emesis. Jpn J Pharmacol. 1993; 63:377–383. PMID: 8107329.
Article16. Yamada Y, Fujita M, Okuyama K, Takayanagi R, Ozeki T, Yokoyama H, et al. Analysis of antiemetic effect of various dosage regimens of azasetron hydrochloride based on 5-HT3 receptor occupancy of serotonin. Yakugaku Zasshi. 2007; 127:353–357. PMID: 17268155.
Article17. Perez EA. Review of the preclinical pharmacology and comparative efficacy of 5-hydroxytryptamine-3 receptor antagonists for chemotherapy-induced emesis. J Clin Oncol. 1995; 13:1036–1043. PMID: 7707101.
Article18. Tsukuda M, Mochimatsu I, Furukawa M, Kohno H, Kawai S, Enomoto H, et al. A randomized crossover comparison of azasetron and granisetron in the prophylaxis of emesis induced by chemotherapy including cisplatin. Gan To Kagaku Ryoho. 1995; 22:1959–1967. PMID: 7487127.19. Kimura E, Niimi S, Watanabe A, Akiyama M, Tanaka T. Study on clinical effect of a continuous intravenous infusion of azasetron against nausea and vomiting induced by anticancer drugs including CDDP. Gan To Kagaku Ryoho. 1996; 23:477–481. PMID: 8678501.20. Navari R, Gandara D, Hesketh P, Hall S, Mailliard J, Ritter H, et al. The Granisetron Study Group. Comparative clinical trial of granisetron and ondansetron in the prophylaxis of cisplatin-induced emesis. J Clin Oncol. 1995; 13:1242–1248. PMID: 7738628.21. Latreille J, Pater J, Johnston D, Laberge F, Stewart D, Rusthoven J, et al. National Cancer Institute of Canada Clinical Trials Group. Use of dexamethasone and granisetron in the control of delayed emesis for patients who receive highly emetogenic chemotherapy. J Clin Oncol. 1998; 16:1174–1178. PMID: 9508205.22. Pater JL, Lofters WS, Zee B, Dempsey E, Walde D, Moquin JP, et al. The role of the 5-HT3 antagonists ondansetron and dolasetron in the control of delayed onset nausea and vomiting in patients receiving moderately emetogenic chemotherapy. Ann Oncol. 1997; 8:181–185. PMID: 9093728.23. The Italian Group for Antiemetic Research. Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med. 2000; 342:1554–1559. PMID: 10824073.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ondansetron compared with ondansetron plus metoclopramide in the prevention of cisplatin-induced emesis
- Comparison of Azasetron and Ondansetron for Preventing Postoperative Nausea and Vomiting in Patients Undergoing Gynecological Laparoscopic Surgery
- Comparative study of an aprepitant regimen with an ondansetron regimen, for efficacy in gynecological cancer patients with chemotherapy
- Ondansetron Reduces Postoperative Nausea and Vomiting after a Laparoscopic Cholecystectomy
- Comparative Study of an Ondansetron and a Ramosetron an Aprepitant in the Control of Nausea and Vomiting in Gynecologinc Cancer Patient with Chemotherapy