Cancer Res Treat.
2013 Dec;45(4):354-358.
Sarcoidosis Mimicking Cancer Metastasis Following Chemotherapy for Ovarian Cancer
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea. jubilate@pusan.ac.kr
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- 3Department of Obstetrics and Gynecology, Pusan National University School of Medicine, Busan, Korea.
Abstract
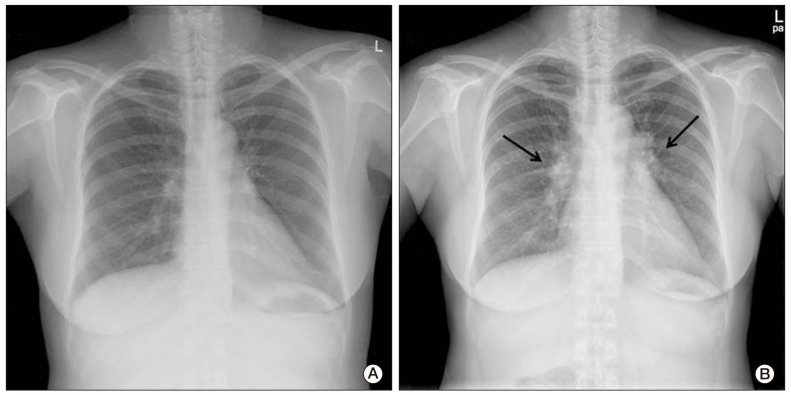
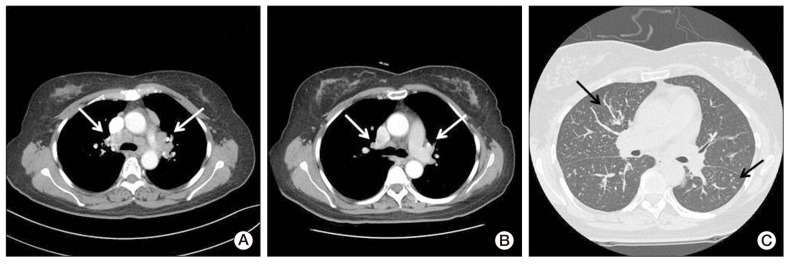
- We report on a rare case of sarcoidosis that developed after chemotherapy for ovarian cancer, and mimicked a cancer metastasis. A 52-year-old female diagnosed with stage III ovarian cancer underwent curative surgery and postoperative chemotherapy. Four months later, her whole-body positron emission tomography and computed tomography (CT) scan showed high uptake in the mediastinal lymph nodes, and ovarian cancer recurrence was suspected. Biopsy of the mediastinal lymph nodes and subcutaneous nodules revealed noncaseating granulomas. These lesions resolved spontaneously without treatment; however, newly developed perilymphatic and centrilobular nodules were observed on follow-up chest CT. Surgical biopsy of these lesions also showed noncaseating granulomas. She was finally diagnosed with sarcoidosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997; 336:1224–1234. PMID: 9110911.
Article2. Caras WE, Dillard T, Baker T, Pluss J. Coexistence of sarcoidosis and malignancy. South Med J. 2003; 96:918–922. PMID: 14513992.
Article3. Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995; 107:605–613. PMID: 7874925.
Article4. Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974; 29:247–251. PMID: 4830144.
Article5. Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev. 1986; 13:147–156. PMID: 3536088.
Article6. Llombart A Jr, Escudero JM. The incidence and significance of epithelioid and sarcoid-like cellular reaction in the stromata of malignant tumours:a morphological and experimental study. Eur J Cancer. 1970; 6:545–551. PMID: 5504464.7. Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007; 25:326–333. PMID: 17560310.
Article8. Tangen JM, Naess A, Aasen T, Morild I. Non-caseating granulomas in patients with hematologic malignancies: a report of three cases. Acta Med Scand. 1988; 223:83–87. PMID: 3126625.9. Kornacker M, Kraemer A, Leo E, Ho AD. Occurrence of sarcoidosis subsequent to chemotherapy for non-Hodgkin's lymphoma: report of two cases. Ann Hematol. 2002; 81:103–105. PMID: 11907791.
Article10. Sybert A, Butler TP. Sarcoidosis following adjuvant high-dose methotrexate therapy for osteosarcoma. Arch Intern Med. 1978; 138:488–489. PMID: 272865.
Article11. Whittington R, Lazarus A, Nerenstone S, Martin A. Sarcoidosis developing during therapy for breast cancer. Chest. 1986; 89:762–763. PMID: 3009099.
Article12. Sacchi S, Kantarjian H, O'Brien S, Cohen PR, Pierce S, Talpaz M. Immune-mediated and unusual complications during interferon alfa therapy in chronic myelogenous leukemia. J Clin Oncol. 1995; 13:2401–2407. PMID: 7666100.
Article13. Umezu H, Chida M, Inoue T, Araki O, Tamura M, Tatewaki M, et al. Sarcoidosis development during induction chemotherapy for lung cancer mimicked progressive disease. Gen Thorac Cardiovasc Surg. 2010; 58:434–437. PMID: 20703868.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics of Ovarian Metastasis from Colorectal Cancer
- Metastatic colon cancer of an ovarian cancer origin mimicking primary colon cancer: A case report
- Discovery of Splenic Sarcoidosis Concurrent with the Diagnosis of Ovarian Cancer: A Case Report
- Early port-site metastasis during neoadjuvant chemotherapy in advanced stage ovarian cancer: report of two cases
- Ovarian metastasis from pulmonary adenocarcinoma