J Rheum Dis.
2016 Apr;23(2):130-135. 10.4078/jrd.2016.23.2.130.
Discovery of Splenic Sarcoidosis Concurrent with the Diagnosis of Ovarian Cancer: A Case Report
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea. sglee@pnuh.co.kr
- 2Department of Obstetrics and Gynecology, Pusan National University School of Medicine, Busan, Korea.
- 3Division of Hemato-oncology, Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea.
- 4Division of Rheumatology, Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea.
- KMID: 2222791
- DOI: http://doi.org/10.4078/jrd.2016.23.2.130
Abstract
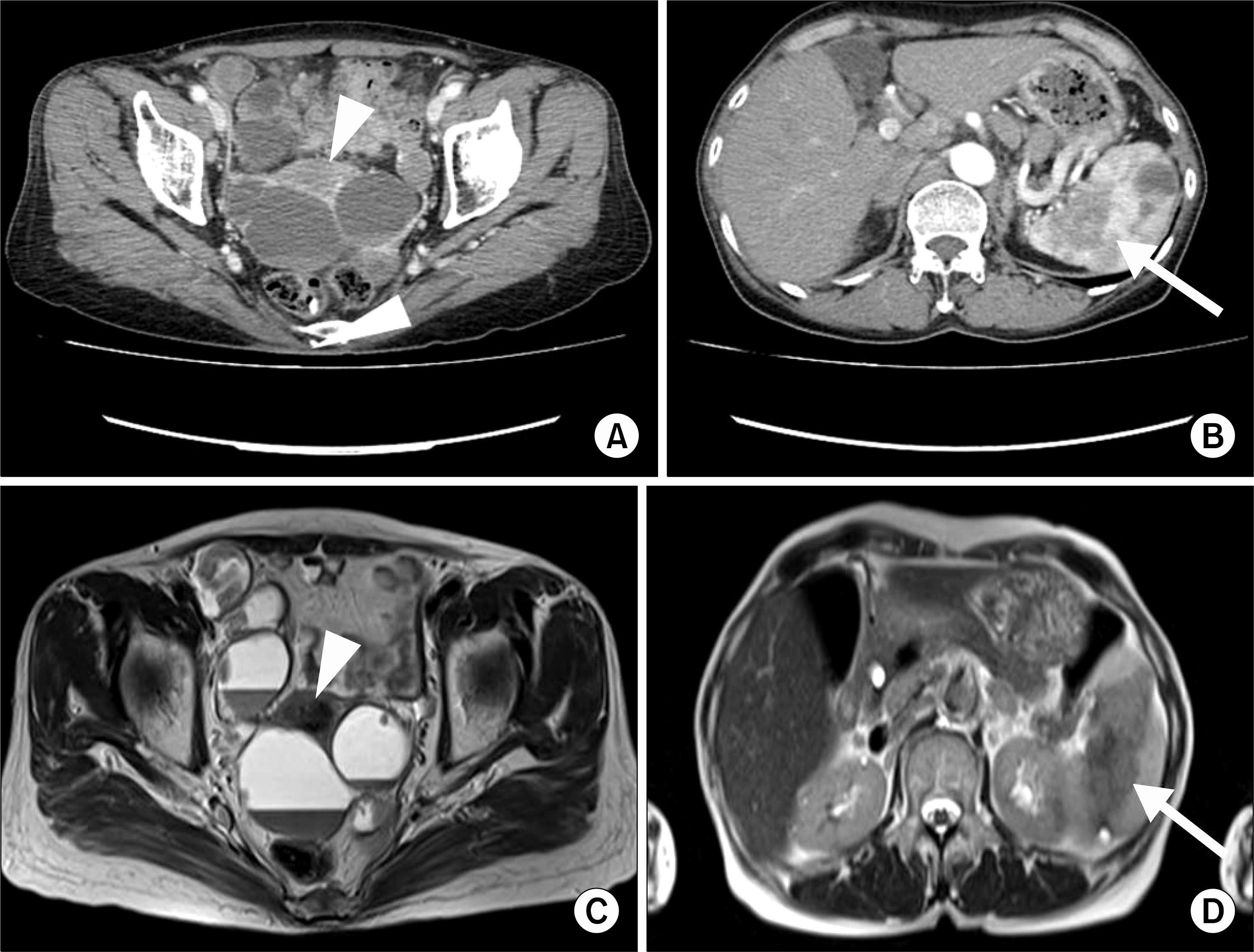
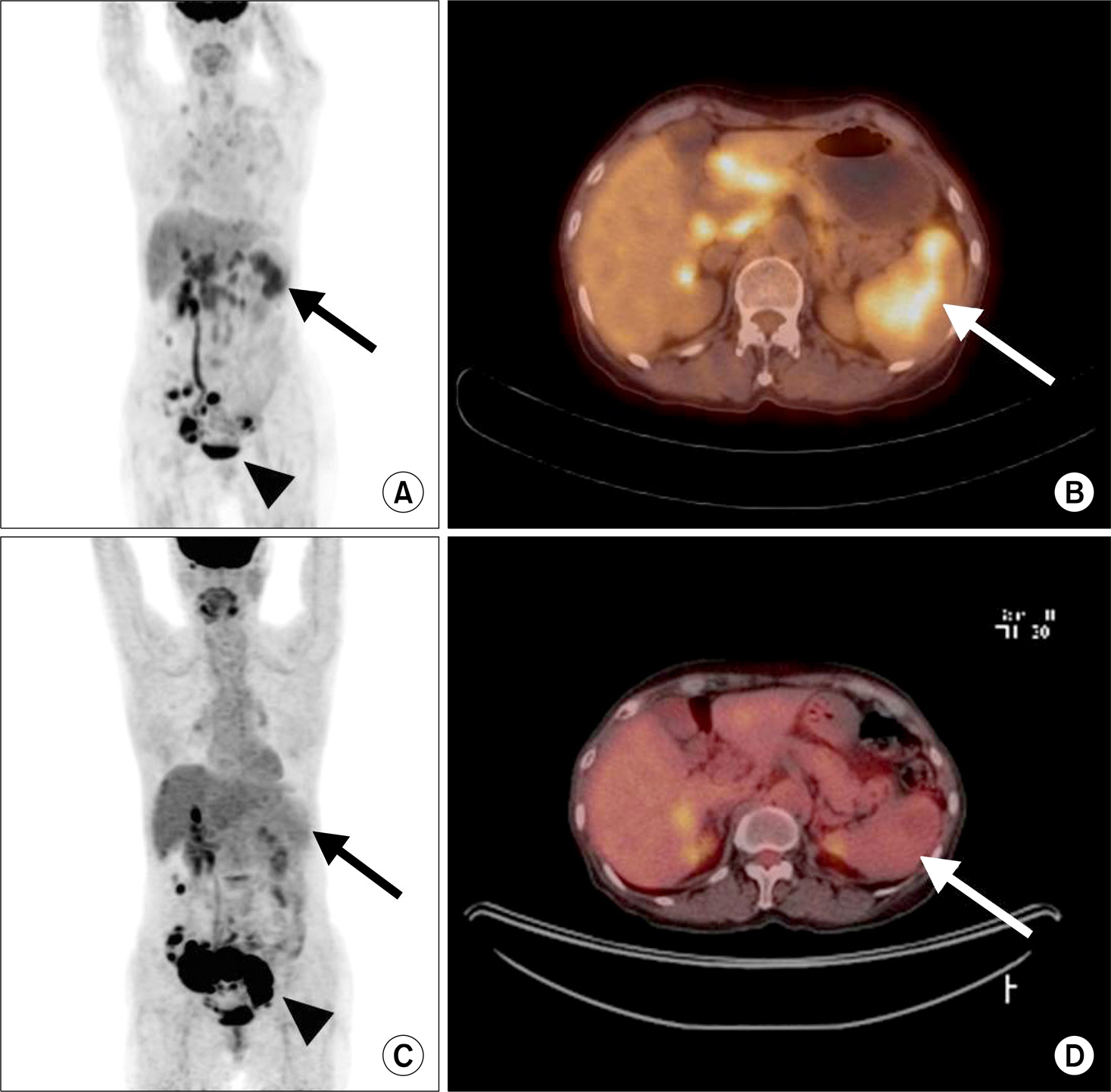
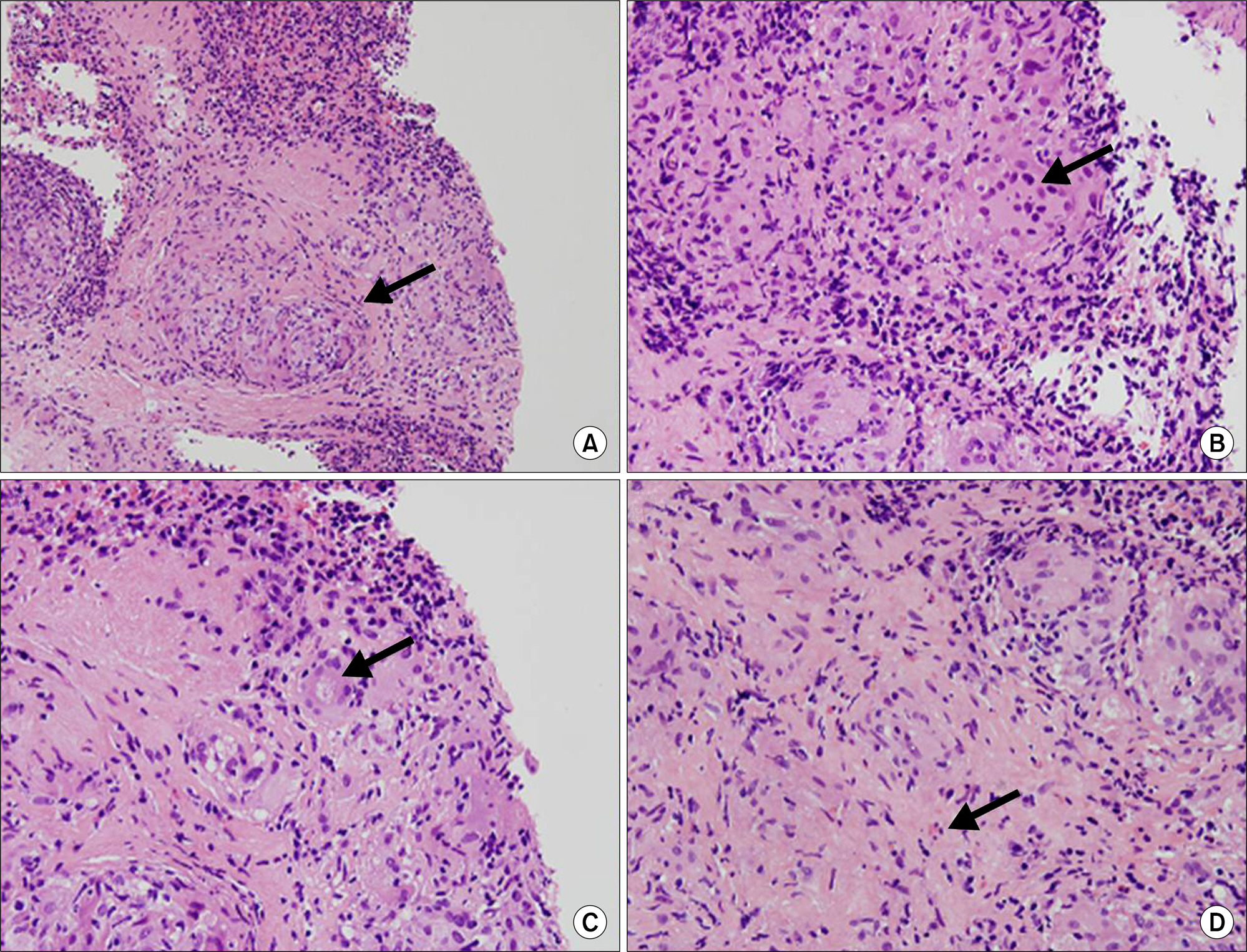
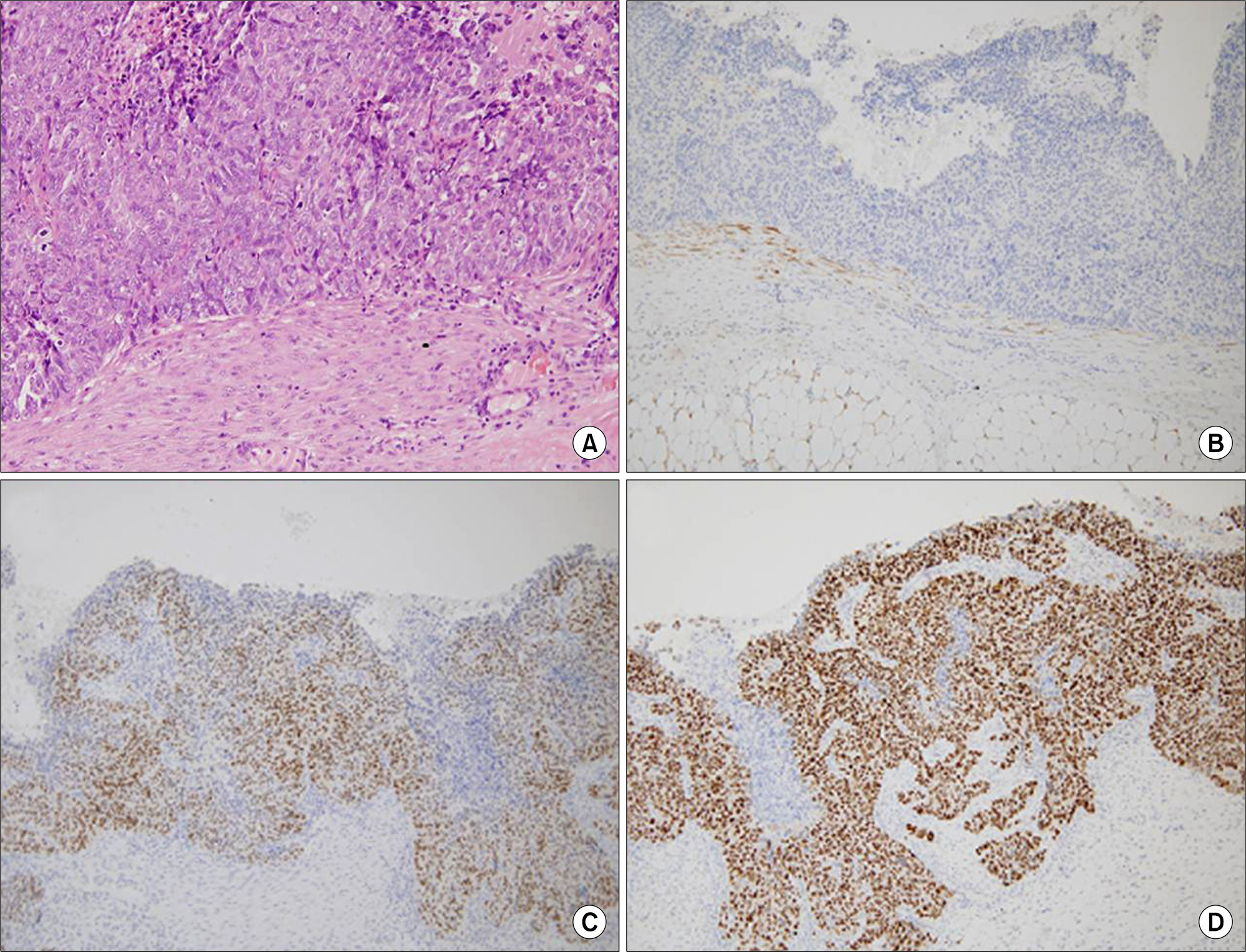
- Sarcoidosis is a multisystem inflammatory disease of unknown etiology characterized by noncaseating epithelioid granuloma formation. Although the relationship between sarcoidosis and malignancy has been noted in recent decades, there are few case reports describing the concurrent diagnosis of sarcoidosis and malignancy. Herein, we describe a case of biopsy-proven splenic sarcoidosis mimicking metastasis at the time of ovarian adenocarcinoma. Imaging studies including positron-emission tomography-computed tomography were not useful for differentiating sarcoidosis from malignancy. Thus, our case highlights the importance of histopathological examination to rule out nonmalignant conditions before the diagnosis of metastatic disease is made.
MeSH Terms
Figure
Reference
-
1. Brincker H, Wilbek E. The incidence of malignant tumors in patients with sarcoidosis. Ugeskr Laeger. 1974; 136:2192–5.2. Reich JM, Mullooly JP, Johnson RE. Linkage analysis of ma-lignancy-associated sarcoidosis. Chest. 1995; 107:605–13.
Article3. Rømer FK, Hommelgaard P, Schou G. Sarcoidosis and cancer revisited: a long-term follow-up study of 555 Danish sarcoidosis patients. Eur Respir J. 1998; 12:906–12.
Article4. Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007; 25:326–33.
Article5. Askling J, Grunewald J, Eklund A, Hillerdal G, Ekbom A. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999; 160:1668–72.
Article6. Seersholm N, Vestbo J, Viskum K. Risk of malignant neoplasms in patients with pulmonary sarcoidosis. Thorax. 1997; 52:892–4.
Article7. Kim MH, Lee K, Kim KU, Park HK, Lee MK, Suh DS. Sarcoidosis mimicking cancer metastasis following chemotherapy for ovarian cancer. Cancer Res Treat. 2013; 45:354–8.
Article8. Logan TF, Bensadoun ES. Increased disease activity in a patient with sarcoidosis after high dose interleukin 2 treatment for metastatic renal cancer. Thorax. 2005; 60:610–1.
Article9. Tolaney SM, Colson YL, Gill RR, Schulte S, Duggan MM, Shulman LN, et al. Sarcoidosis mimicking metastatic breast cancer. Clin Breast Cancer. 2007; 7:804–10.
Article10. Lequoy M, Coriat R, Rouquette A, Mir O, Perkins G, Regnard JF, et al. Sarcoidosis lung nodules in colorectal cancer follow-up: sarcoidosis or not? Am J Med. 2013; 126:642–5.
Article11. Jiao Y, Ning J, Zhao WD, Li YL, Wu HY, Gu KS. Sarcoidosis in gastric cancer at the time of diagnosis: a case report. Oncol Lett. 2015; 9:1159–62.
Article12. Mapelli P, Mangili G, Picchio M, Rabaiotti E, Gianolli L, Messa C, et al. Sarcoidosis mimicking metastatic gynaeco-logical malignancies: a diagnostic and therapeutic chal-lenge? Rev Esp Med Nucl Imagen Mol. 2013; 32:314–7.
Article13. Idali F, Wahlström J, Müller-Suur C, Eklund A, Grunewald J. Analysis of regulatory T cell associated forkhead box P3 expression in the lungs of patients with sarcoidosis. Clin Exp Immunol. 2008; 152:127–37.
Article14. Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006; 203:359–70.
Article15. Tsiatas ML, Gyftaki R, Liacos C, Politi E, Rodolakis A, Dimopoulos MA, et al. Study of T lymphocytes infiltrating peritoneal metastases in advanced ovarian cancer: associations with vascular endothelial growth factor levels and prognosis in patients receiving platinum-based chemotherapy. Int J Gynecol Cancer. 2009; 19:1329–34.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Ichthyosiform Sarcoidosis
- Sarcoidosis Mimicking Cancer Metastasis Following Chemotherapy for Ovarian Cancer
- Sarcoidosis in a Four-year-old Girl
- A Case of Multiple Primary Cancer with Cervical Adenocarcinoma and both Ovarian Endometrioid Adenocarcinoma
- Sonographic Appearance of Dermal and Subcutaneous Sarcoidosis: A Case Report