Allergy Asthma Immunol Res.
2011 Oct;3(4):245-250. 10.4168/aair.2011.3.4.245.
Assessment of Bronchodilator Responsiveness Following Methacholine-Induced Bronchoconstriction in Children With Asthma
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Korea University, Seoul, Korea. yoolina@korea.ac.kr
- 2Department of Pediatrics, Geoje Baek Hospital, Geoje, Korea.
- KMID: 2167075
- DOI: http://doi.org/10.4168/aair.2011.3.4.245
Abstract
- PURPOSE
The aim of this study was to investigate bronchodilator responsiveness (BDR) following methacholine-induced bronchoconstriction and to determine differences in BDR according to clinical parameters in children with asthma.
METHODS
The methacholine challenge test was performed in 145 children with mild to moderate asthma, and the provocative concentration causing a 20% decline in FEV1 (PC20) was determined. Immediately after the challenge test, patients were asked to inhale short-acting beta2-agonists (SABAs) to achieve BDR, which was assessed as the change in FEV1% predictedx100/post-methacholine FEV1% predicted. For each subject, the asthma medication, blood eosinophil count, serum total IgE, serum eosinophil cationic protein level, and skin prick test result were assessed.
RESULTS
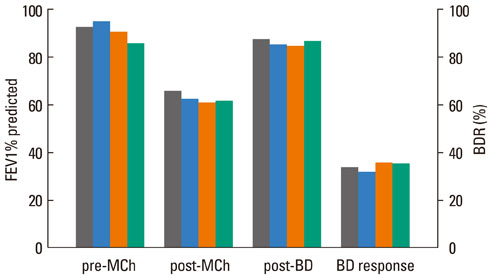
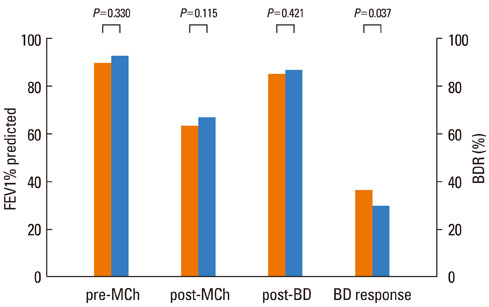
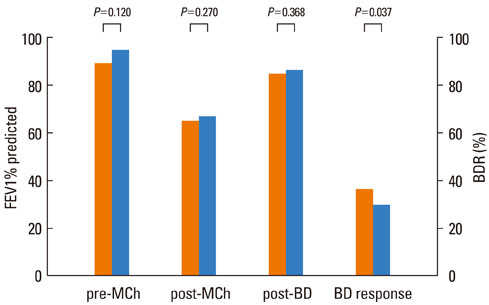
The FEV1 (mean+/-SD) values of the 145 patients were 90.5+/-10.9% predicted, 64.2+/-11.5% predicted, and 86.2+/-11.2% predicted before and after methacholine inhalation, and following the administration of a SABA, respectively. The BDR did not differ significantly according to asthma medication, age, or gender. However, BDR in the atopy group (37.4+/-17.7%) was significantly higher than that in the non-atopy group (30.5+/-10.7%; P=0.037). Patients with blood eosinophilia (38.6+/-18.1%) displayed increased BDR compared with patients without eosinophilia (32.0+/-13.8%; P=0.037).
CONCLUSIONS
In children with mild to moderate asthma, the responsiveness to short-acting bronchodilators after methacholine-induced bronchoconstriction was not related to asthma medication, but was higher in children with atopy and/or peripheral blood eosinophilia.
Keyword
MeSH Terms
-
Adrenergic beta-Agonists
Asthma
Azides
Bronchoconstriction
Bronchodilator Agents
Child
Eosinophil Cationic Protein
Eosinophilia
Eosinophils
Humans
Immunoglobulin E
Inhalation
Methacholine Chloride
Serotonin
Skin
Adrenergic beta-Agonists
Azides
Bronchodilator Agents
Eosinophil Cationic Protein
Immunoglobulin E
Methacholine Chloride
Serotonin
Figure
Cited by 1 articles
-
Usefulness of bronchodilator response as an index of asthma control in children
Jong Deok Kim, Soo Yeon Kim, Yoon Hee Kim, Kyung Won Kim, Myung Hyun Sohn, In Suk Sol
Allergy Asthma Respir Dis. 2019;7(2):92-98. doi: 10.4168/aard.2019.7.2.92.
Reference
-
1. Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004. 59:469–478.2. Bousquet J, Clark TJ, Hurd S, Khaltaev N, Lenfant C, O'Byrne P, Sheffer A. GINA guidelines on asthma and beyond. Allergy. 2007. 62:102–112.3. Malakauskas K, Sitkauskiene B, Stravinskaite K, Sakalauskas R. Dyspnea perception and reversibility of methacholine-induced unlimited airway narrowing in asthmatics. J Asthma. 2006. 43:463–467.4. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008. 31:143–178.5. Abramson MJ, Walters J, Walters EH. Adverse effects of beta-agonists: are they clinically relevant? Am J Respir Med. 2003. 2:287–297.6. Juniper EF, Frith PA, Hargreave FE. Airway responsiveness to histamine and methacholine: relationship to minimum treatment to control symptoms of asthma. Thorax. 1981. 36:575–579.7. Fourie PR, Joubert JR. Determination of airway hyper-reactivity in asthmatic children: a comparison among exercise, nebulized water, and histamine challenge. Pediatr Pulmonol. 1988. 4:2–7.8. Smith L, McFadden ER Jr. Bronchial hyperreactivity revisited. Ann Allergy Asthma Immunol. 1995. 74:454–469.9. Parker AL. Airway reactivity is a determinant of bronchodilator responsiveness after methacholine-induced bronchoconstriction. J Asthma. 2004. 41:671–677.10. Haney S, Hancox RJ. Recovery from bronchoconstriction and bronchodilator tolerance. Clin Rev Allergy Immunol. 2006. 31:181–196.11. National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma: expert panel report 2. 1997. Bethesda: US Dept of Health and Human Services;(NIH publication; no. 97-4051).12. American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995. 152:1107–1136.13. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, Sheffer AL, Spector SL, Townley RG. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975. 56:323–327.14. Dales RE, Spitzer WO, Tousignant P, Schechter M, Suissa S. Clinical interpretation of airway response to a bronchodilator. Epidemiologic considerations. Am Rev Respir Dis. 1988. 138:317–320.15. Smith AP, Orehek J, Charpin J. Bronchodilator drug tests in normal subjects [proceedings]. Br J Dis Chest. 1977. 71:234–236.16. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000. 161:309–329.17. Parker AL. Aging does not affect beta-agonist responsiveness after methacholine-induced bronchoconstriction. J Am Geriatr Soc. 2004. 52:388–392.18. van der Woude HJ, Winter TH, Aalbers R. Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting β2 agonists. Thorax. 2001. 56:529–535.19. Waalkens HJ, Merkus PJ, van Essen-Zandvliet EE, Brand PL, Gerritsen J, Duiverman EJ, Kerrebijn KF, Knol KK, Quanjer PH. Dutch CNSLD Study Group. Assessment of bronchodilator response in children with asthma. Eur Respir J. 1993. 6:645–651.20. Brand PL, Quanjer PH, Postma DS, Kerstjens HA, Koeter GH, Dekhuijzen PN, Sluiter HJ. Dutch Chronic Non-Specific Lung Disease (CNSLD) Study Group. Interpretation of bronchodilator response in patients with obstructive airways disease. Thorax. 1992. 47:429–436.21. Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator responsiveness in wheezy children. Thorax. 2005. 60:13–16.22. Hancox RJ, Aldridge RE, Cowan JO, Flannery EM, Herbison GP, McLachlan CR, Town GI, Taylor DR. Tolerance to beta-agonists during acute bronchoconstriction. Eur Respir J. 1999. 14:283–287.23. Merkus PJ, Rooda HM, van Essen-Zandvliet EE, Duiverman EJ, Quanjer PH, Kerrebijn KF. Assessment of bronchodilatation after spontaneous recovery from a histamine challenge in asthmatic children. Thorax. 1992. 47:355–359.24. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005. 26:948–968.25. Kumar R, Wang B, Wang X, Chen C, Yang J, Fu L, Xu X. Bronchodilator responses in Chinese children from asthma index families and the general population. J Allergy Clin Immunol. 2006. 117:1257–1263.26. Zietkowski Z, Bodzenta-Lukaszyk A, Tomasiak MM, Skiepko R, Szmitkowski M. Comparison of exhaled nitric oxide measurement with conventional tests in steroid-naïve asthma patients. J Investig Allergol Clin Immunol. 2006. 16:239–246.27. Silvestri M, Sabatini F, Sale R, Defilippi AC, Fregonese L, Battistini E, Biraghi MG, Rossi GA. Correlations between exhaled nitric oxide levels, blood eosinophilia, and airway obstruction reversibility in childhood asthma are detectable only in atopic individuals. Pediatr Pulmonol. 2003. 35:358–363.28. Faul JL, Demers EA, Burke CM, Poulter LW. Alterations in airway inflammation and lung function during corticosteroid therapy for atopic asthma. Chest. 2002. 121:1414–1420.29. Sitkauskiene B, Sakalauskas R, Malakauskas K, Lötvall J. Reversibility to a β2-agonist in COPD: relationship to atopy and neutrophil activation. Respir Med. 2003. 97:591–598.30. Tantisira KG, Fuhlbrigge AL, Tonascia J, Van Natta M, Zeiger RS, Strunk RC, Szefler SJ, Weiss ST; Childhood Asthma Management Program Research Group. Bronchodilation and bronchoconstriction: predictors of future lung function in childhood asthma. J Allergy Clin Immunol. 2006. 117:1264–1271.31. Djukanović R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, Holgate ST. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990. 142:434–457.32. Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, Sears MR. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002. 165:1480–1488.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of Bronchodilator Responsiveness after Methacholine-Induced Bronchoconstriction
- The effect of deep inspiration on methacholine induced bronchoconstriction
- Differences of Ventilatory Function Measurements between Natural and Methacholine or Histamine-induced Bronchoconstriction in Asthma
- Exercise induced delayed bronchoconstriction in children with asthma
- Significant changes of bronchial responsiveness to methacholine after early asthmatic reaction to toluene diisocyanate (TDI) in a TDI-sensitive asthmatic worker