Allergy Asthma Immunol Res.
2012 May;4(3):157-160. 10.4168/aair.2012.4.3.157.
Changes in Major Peanut Allergens Under Different pH Conditions
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. snuhan@skku.edu
- 2Environmental Health Center for Atopic Diseases, Samsung Medical Center, Seoul, Korea.
- 3Department of Pediatrics, Korea University College of Medicine, Seoul, Korea.
- KMID: 2167068
- DOI: http://doi.org/10.4168/aair.2012.4.3.157
Abstract
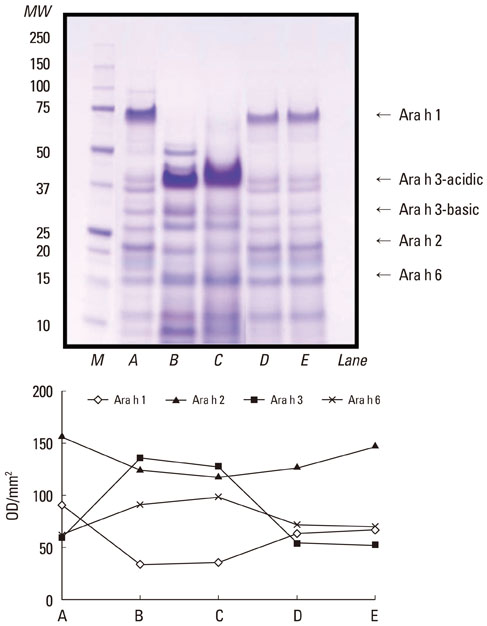
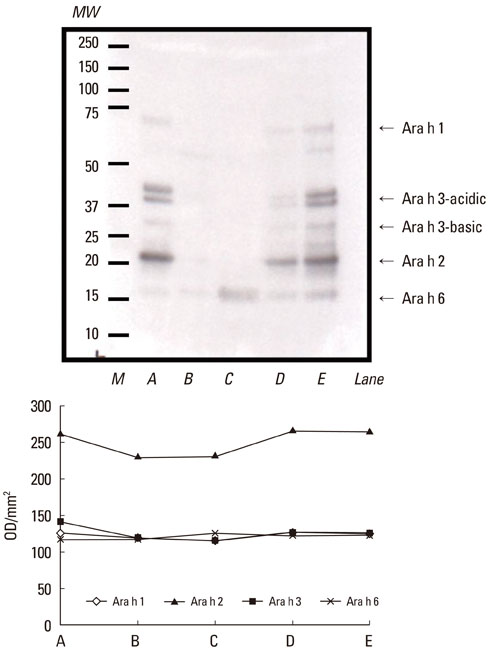
- Regional dietary habits and cooking methods affect the prevalence of specific food allergies; therefore, we determined the effects of various pH conditions on major peanut allergens. Peanut kernels were soaked overnight in commercial vinegar (pH 2.3) or acetic acid solutions at pH 1.0, 3.0, or 5.0. Protein extracts from the sera of seven patients with peanut-specific IgE levels >15 kUA/L were analyzed by SDS-PAGE and immunolabeling. A densitometer was used to quantify and compare the allergenicity of each protein. The density of Ara h 1 was reduced by treatment with pH 1.0, 3.0, or 5.0 acetic acid, or commercial vinegar. Ara h 2 remained largely unchanged after treatment with pH 5.0 acetic acid, and was decreased following treatment with pH 1.0, 2.3, or 3.0 acetic acid. Ara h 3 and Ara h 6 appeared as a thick band after treatment with pH 1.0 acetic acid and commercial vinegar. IgE-binding intensities to Ara h 1, Ara h 2, and Ara h 3 were significantly reduced after treatment with pH 1.0 acetic acid or commercial vinegar. These data suggest that treatment with acetic acid at various pH values affects peanut allergenicity and may explain the low prevalence of peanut allergy in Korea.
MeSH Terms
Figure
Cited by 1 articles
-
How Different Parts of the World Provide New Insights Into Food Allergy
Elizabeth Huiwen Tham, Donald Y.M. Leung
Allergy Asthma Immunol Res. 2018;10(4):290-299. doi: 10.4168/aair.2018.10.4.290.
Reference
-
1. Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004. 113:805–819.2. Piromrat K, Chinratanapisit S, Trathong S. Anaphylaxis in an emergency department: a 2-year study in a tertiary-care hospital. Asian Pac J Allergy Immunol. 2008. 26:121–128.3. Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007. 120:491–503.4. Beyer K, Morrow E, Li XM, Bardina L, Bannon GA, Burks AW, Sampson HA. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001. 107:1077–1081.5. Hill DJ, Hosking CS, Heine RG. Clinical spectrum of food allergy in children in Australia and South-East Asia: identification and targets for treatment. Ann Med. 1999. 31:272–281.6. Chung SY, Butts CL, Maleki SJ, Champagne ET. Linking peanut allergenicity to the processes of maturation, curing, and roasting. J Agric Food Chem. 2003. 51:4273–4277.7. Maleki SJ, Viquez O, Jacks T, Dodo H, Champagne ET, Chung SY, Landry SJ. The major peanut allergen, Ara h 2, functions as a trypsin inhibitor, and roasting enhances this function. J Allergy Clin Immunol. 2003. 112:190–195.8. Ahn YH, Yeo JS, Lee JY, Han YS, Ahn KM, Lee SI. Effects of cooking methods on peanut allergenicity. Pediatr Allergy Respir Dis. 2009. 19:233–240.9. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001. 107:891–896.10. Koppelman SJ, Vlooswijk RA, Knippels LM, Hessing M, Knol EF, van Reijsen FC, Bruijnzeel-Koomen CA. Quantification of major peanut allergens Ara h 1 and Ara h 2 in the peanut varieties Runner, Spanish, Virginia, and Valencia, bred in different parts of the world. Allergy. 2001. 56:132–137.11. Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, Ma S, Lee BW. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010. 126:324–331. 331.e1–331.e7.12. Leung TF, Yung E, Wong YS, Lam CW, Wong GW. Parent-reported adverse food reactions in Hong Kong Chinese pre-schoolers: epidemiology, clinical spectrum and risk factors. Pediatr Allergy Immunol. 2009. 20:339–346.13. Maleki SJ, Chung SY, Champagne ET, Raufman JP. The effects of roasting on the allergenic properties of peanut proteins. J Allergy Clin Immunol. 2000. 106:763–768.14. Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy. 2004. 34:583–590.15. Astier C, Morisset M, Roitel O, Codreanu F, Jacquenet S, Franck P, Ogier V, Petit N, Proust B, Moneret-Vautrin DA, Burks AW, Bihain B, Sampson HA, Kanny G. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. J Allergy Clin Immunol. 2006. 118:250–256.16. Peeters KA, Koppelman SJ, van Hoffen E, van der Tas CW, den Hartog Jager CF, Penninks AH, Hefle SL, Bruijnzeel-Koomen CA, Knol EF, Knulst AC. Does skin prick test reactivity to purified allergens correlate with clinical severity of peanut allergy? Clin Exp Allergy. 2007. 37:108–115.17. McDermott RA, Porterfield HS, El Mezayen R, Burks AW, Pons L, Schlichting DG, Solomon B, Redzic JS, Harbeck RJ, Duncan MW, Hansen KC, Dreskin SC. Contribution of Ara h 2 to peanut-specific, immunoglobulin E-mediated, cell activation. Clin Exp Allergy. 2007. 37:752–763.18. Blanc F, Adel-Patient K, Drumare MF, Paty E, Wal JM, Bernard H. Capacity of purified peanut allergens to induce degranulation in a functional in vitro assay: Ara h 2 and Ara h 6 are the most efficient elicitors. Clin Exp Allergy. 2009. 39:1277–1285.19. Porterfield HS, Murray KS, Schlichting DG, Chen X, Hansen KC, Duncan MW, Dreskin SC. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variants. Clin Exp Allergy. 2009. 39:1099–1108.20. Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, Härlin A, Woodcock A, Ahlstedt S, Custovic A. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010. 125:191–197.e1-13.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Anaphylaxis Induced by Peanut
- Clinical Significance of Component Allergens in Fagales Pollen-Sensitized Peanut Allergy in Korea
- A comparison study on allergen components between Korean (Arachis fastigiata Shinpung) and American peanut (Arachis hypogaea Runner)
- Effects of Cooking Methods on Peanut Allergenicity
- Prevention of food allergy in infants: recommendation for infant feeding and complementary food introduction