Cancer Res Treat.
2009 Mar;41(1):12-18.
Randomized, Multicenter, Phase III Trial of Heptaplatin 1-hour Infusion and 5-Fluorouracil Combination Chemotherapy Comparing with Cisplatin and 5-Fluorouracil Combination Chemotherapy in Patients with Advanced Gastric Cancer
- Affiliations
-
- 1Korea Cancer Study Group, Korea.
- 2College of Medicine, Yeungnam University, Daegu, Korea.
- 3College of Medicine, The Catholic University of Korea, St. Vincent's Hospital, Suwon, Korea. kimhoonkyo@yahoo.co.kr
- 4College of Medicine, Keimyung University, Dongsan Medical Center, Daegu, Korea.
- 5College of Medicine, Daegu Catholic University, Daegu, Korea.
- 6College of Medicine, Pusan National University, Busan, Korea.
- 7College of Medicine, Hallym Sacred Heart Hospital, Seoul, Korea.
- 8College of Medicine, Sungkyunkwan University, Samsung Medical Center, Seoul, Korea.
- 9College of Medicine, The Catholic University of Korea, Holy Family Hospital, Bucheon, Korea.
- 10College of Medicine, Chonbuk National University, Chonbuk, Korea.
- 11College of Medicine, Soon Chun Hyang University, Seoul, Korea.
- 12College of Medicine, Korea University, Guro Hospital, Seoul, Korea.
- 13College of Medicine, Inje University Pusan Paik Hospital, Busan, Korea.
- 14College of Medicine, Ewha Womans University, Seoul, Korea.
Abstract
-
PURPOSE: Heptaplatin (Sunpla) is a cisplatin derivative. A phase IIb trial using heptaplatin resulted in a 34% response rate with mild nephrotoxicity. We conducted a randomized phase III trial of heptaplatin plus 5-FU compared with cisplatin plus 5-FU in patients with advanced gastric cancer.
MATERIALS AND METHODS
One hundred seventy-four patients (heptaplatin, n=88; cisplatin, n=86) from 13 centers were enrolled. The eligibility criteria were as follows: patients with pathologically-proven adenocarcinoma, chemonaive patients, or patients who had received only single adjuvant chemotherapy, and who had a measurable or evaluable lesion. On day 1, heptaplatin (400 mg/m2) or cisplatin (60 mg/m2) was given over 1 hour with 5-FU (1 gm/m2) on days 1~5 every 4 weeks.
RESULTS
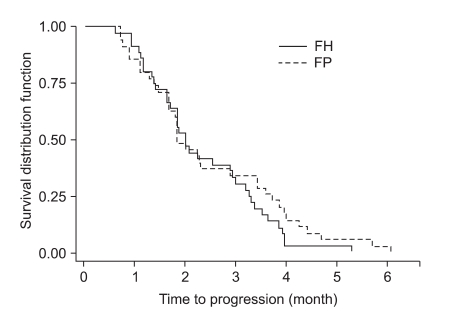
At the time of survival analysis, the median overall survival was 7.3 months in the 5-FU + heptaplatin (FH) arm and 7.9 months in the 5-FU + cisplatin (FP) arm (p=0.24). Of the FH patients, 34.2% (complete response [CR], 1.3%; partial response [PR], 32.9%) experienced a confirmed objective response compared with 35.9% (CR 0%, PR 35.9%) of FP patients (p=0.78). The median-time-to-progression was 2.5 months in the FH arm and 2.3 months in the FP arm. The incidence of neutropenia was higher with FP (28%) than with FH (16%; p=0.06); grade 3~4 nausea and vomiting were more frequent in the FP than in the FH arm (p=0.01 and p=0.05, respectively). The incidence of increased proteinuria and creatininemia was higher with FH than with FP; however, there was no statistical difference. There were no treatment-related deaths.
CONCLUSION
Heptaplatin showed similar effects to cisplatin when combined with 5-FU in advanced gastric cancer patients with tolerable toxicities.
MeSH Terms
Figure
Reference
-
1. Jemal A, Thomas A, Murray T, Thun M. Cancer statistics 2002. CA Cancer J Clin. 2002; 52:23–47. PMID: 11814064.
Article2. Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics 2001. CA Cancer J Clin. 2001; 51:15–36. PMID: 11577478.
Article3. Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, et al. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993; 71:3813–3818. PMID: 8508349.
Article4. Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol. 2003; 21:54–59. PMID: 12506170.
Article5. Fram RJ. Cisplatin and platinum analogues: recent advances. Curr Opin Oncol. 1992; 4:1073–1079. PMID: 1457521.6. Reed E, Dabholkar M, Chabner BA. Chabner BA, Longo DL, editors. Platinum analogues. Cancer chemotherapy and biotherapy: principles and practice. 1996. 2nd. edition. Philadelphia: Lippincott-Raven;p. 357–378.7. Harrap KR. Preclinical studies identifying carboplatin as a visible cisplatin alternative. Cancer Treat Rev. 1985; 12(Suppl A):21–33. PMID: 3910219.8. McKeage MJ, Higgins JD 3rd, Kelland LR. Platinum and other metal coordination compounds in cancer chemotherapy. A commentary on the sixth international symposium, San Diego, Califonia, 23-26th January 1991. Br J Cancer. 1991; 64:788–792. PMID: 1911229.9. Rose WC, Schuring JE. Preclinical antitumor and toxicologic profile of carboplatin. Cancer Treat Rev. 1985; 12(Suppl A):1–19. PMID: 3910215.
Article10. Gore ME, Fryatt I, Wiltshaw E, Dawson T, Robinson BA, Calvert AH. Cisplatin/carboplatin cross-resistance in ovarian cancer. Br J Cancer. 1989; 60:767–769. PMID: 2803953.
Article11. Kim DK, Kim G, Gam J, Cho YB, Kim HT, Tai JH, et al. Synthesis and antitumor activity of a series of [2-sub-stituted-4, 5-bis (aminomethl)-1, 3-dioxolane] platinum(II) complexes. J Med Chem. 1994; 37:1471–1485. PMID: 8182706.12. Kim DK, Kim HT, Cho YB, Tai JH, Ahn JS, Kim TS, et al. Antitumor activity of cis-malonato [(4R, 5R)-4, 5 bis(aminomethy)-2-isopropyl-1,3-dioxolane] platinum(II), a new platinum analogue, as an anticancer agent. Cancer Chemother Pharmacol. 1995; 35:441–445. PMID: 7850928.13. Kim HT, Kim DK, Cho YB, Kim TS, Jung I, Kim KH, et al. Influence of exposure and infusion times on the cytotoxicity and pharmacokinetics of cis-malonato [(4R, 5R)-4,5-bis(aminomethy)-2-isopropyl-1,3-dioxolane] platinum(II). Cancer Chemother Pharmacol. 1998; 41:109–116. PMID: 9443623.14. Kim NK, Im SA, Kim DW, Lee MH, Jung CW, Cho EK, et al. Phase II clinical trial of SKI-2053R, a new platinum analog, in the treatment of patients with advanced gastric adenocarcinoma. Cancer. 1999; 86:1109–1115. PMID: 10506693.
Article15. Min YJ, Bang SJ, Shin JW, Kim DH, Park JH, Kim GY, et al. Combination chemotherapy with 5-fluorouracil and heptaplatin as first-line treatment in patients with advanced gastric cancer. J Korean Med Sci. 2004; 19:369–373. PMID: 15201502.
Article16. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47:207–214. PMID: 7459811.
Article17. Hong WS, Kim HT, Kim KH, Kim DK. In vitro antitumor activity of a new platinum complex, cis-malonato (4R,5R)-4,5-bis(aminomethyl)-1,3-dioxolane-platinum(II) (SKI2053R), against human lung and stomach cancer cell lines. Anticancer Res. 1995; 15:51–54. PMID: 7733640.18. Loehrer PJ, Einhorn LH. Drugs five years later. Cisplatin. Ann Intern Med. 1984; 100:704–713. PMID: 6370067.19. Madias NE, Harrington JT. Platinum nephrotoxicity. Am J Med. 1987; 65:307–314. PMID: 99034.
Article20. Moertel CG, Rubin J, O'Connell MJ, Schutt AJ, Wieand HS. A phase II study of combined 5-fluorouracil, doxorubicin, and cisplatin in the treatment of advanced upper gastrointestinal adenocarcinomas. J Clin Oncol. 1986; 4:1053–1057. PMID: 3014083.
Article21. Kim YH. Chemotherapy for advanced gastric cancer: slow but further progression. Cancer Res Treat. 2005; 37:79–86.22. Ohtsu A, Shimada Y, Yoshida S, Saito H, Seki S, Morise K, et al. Phase II study of protracted infusional 5-flurouracil combined with cisplatinum for advanced gastric cancer: report from the Japan Clinical Oncoloty Group (JCOG). Eur J Cancer. 1994; 30A:2091–2093. PMID: 7857709.23. Gormley PE, Bull JM, Leroy AF, Cysyk R. Kinetics of cis-dichlorodiammineplatinum. Clin Pharmacol Ther. 1979; 25:351–357. PMID: 761445.24. Kim DK, Kim HT, Tai JH, Cho YB, Kim TS, Kim KH, et al. Pharmacokinetics and antitumor activity of a new platinum compound, cis-malonato[(4R,5R)-4,5-bis(aminomethy1)-2isopropy1-1,3-ioxolane]platinum(II) as determined by ex vivo pharmacodynamics. Cancer Chemother pharmacol. 1995; 37:1–6. PMID: 7497577.25. Ahn JH, Kang YK, Kim TW, Bang H, Chang HM, Kang WC, et al. Nephrotoxicity of heptaplatin: a randomized comparion with cisplatin in advanced gastric cancer. Cancer Chemother Pharmacol. 2002; 50:104–110. PMID: 12172973.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combination chemotherapy with 5-fluorouracil and cisplatin for advanced gastric cancer
- Adjuvant Chemotherapy in Gastric Cancer
- 5-Fluorouracil, heptaplatin and UFT combination chemotherapy for advanced or recurrent gastric cancer
- Chemotherapy of Advanced Gastric Cancer
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?