Allergy Asthma Immunol Res.
2016 Mar;8(2):115-123. 10.4168/aair.2016.8.2.115.
Prognostic Factors for Chronic Spontaneous Urticaria: A 6-Month Prospective Observational Study
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 2Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Clinical Trial Center, Ajou University Medical Center, Suwon, Korea.
- KMID: 2165927
- DOI: http://doi.org/10.4168/aair.2016.8.2.115
Abstract
- PURPOSE
Chronic urticaria (CU) has a substantial impact on the quality of life. Little clinical data on the prognosis of CU has been reported. This study aimed to investigate the control status and remission rate of CU and to explore potential predictors of good responses to the treatment during a 6-month treatment period.
METHODS
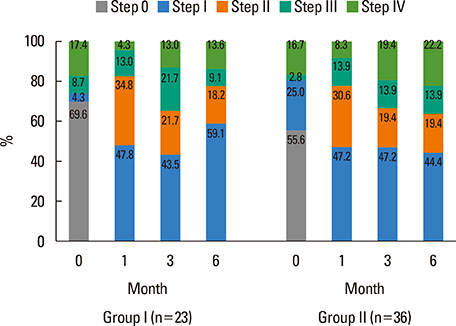
A total of 75 patients with chronic spontaneous urticaria (CSU) were enrolled from 3 university hospitals in Korea. Urticaria control state was classified into 2 groups: group I (remission and well-controlled) and group II (partly and uncontrolled). CU-specific quality of life (CU-QoL) and the urticaria activity score (UAS) were measured before and after the treatment. Autologous serum skin test (ASST), and anti-nuclear and anti-thyroid antibodies were measured at the enrollment into the study. Aspirin intolerance was confirmed by an oral provocation test.
RESULTS
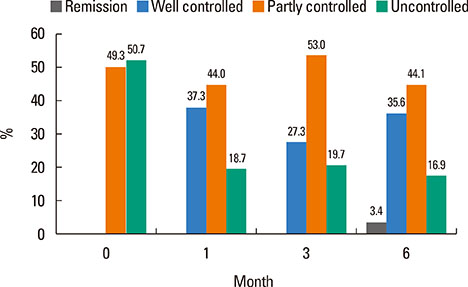
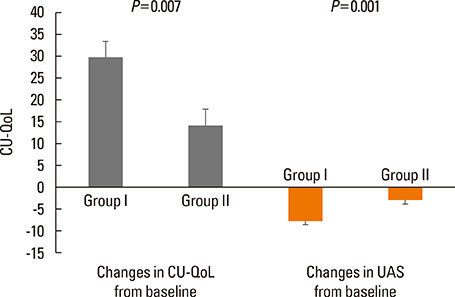
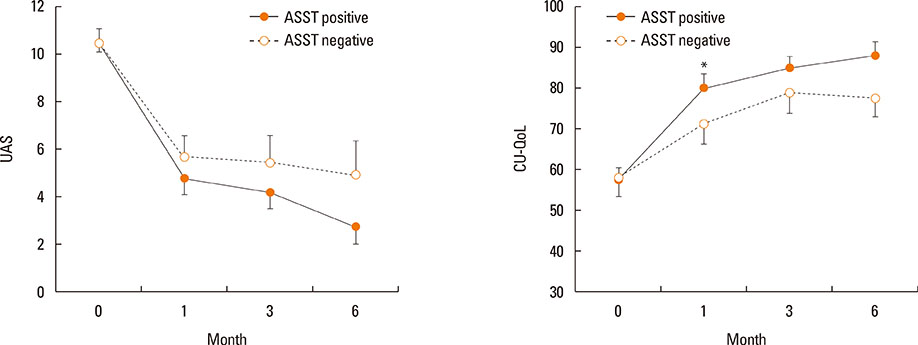
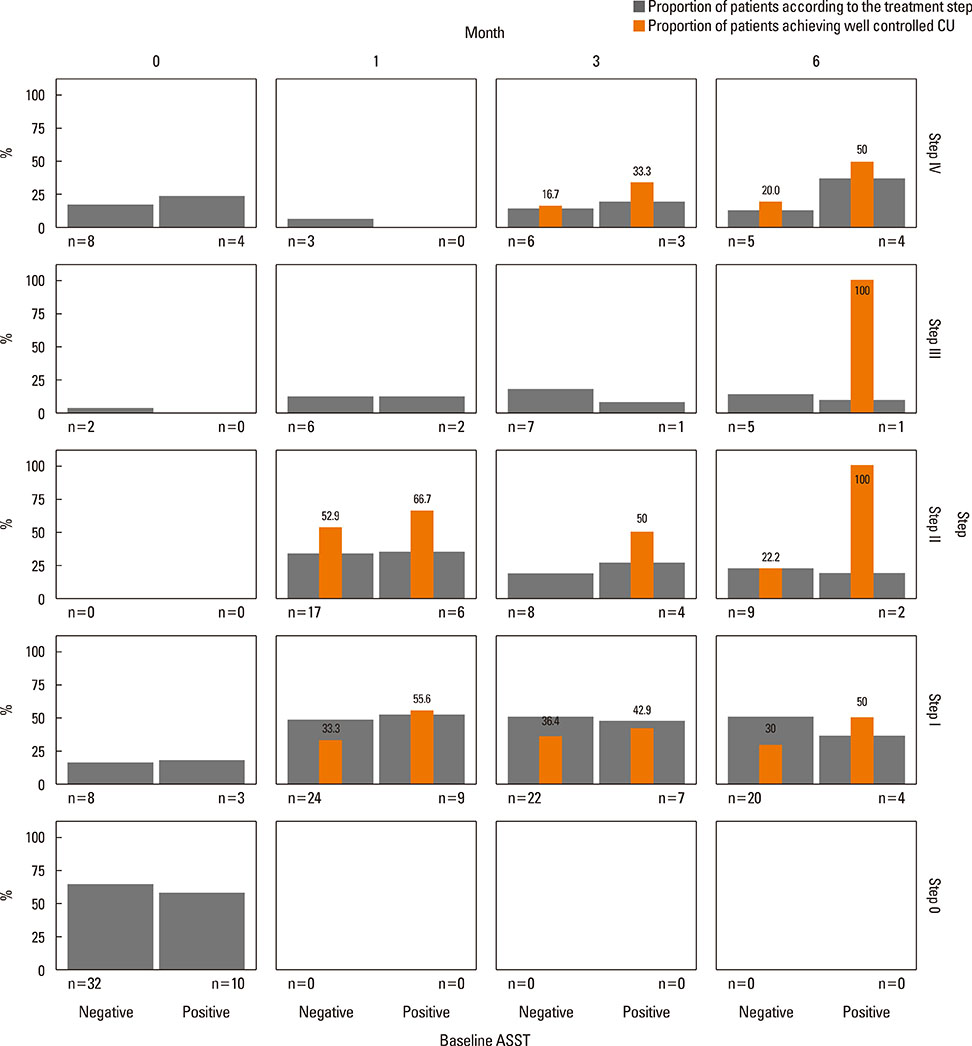
Of 59 patients completing the study, 21 (35.6%) arrived at well-controlled status and only 2 (3.4%) achieved remission, whereas 26 (44.1%) remained at partly controlled status and 10 (16.9%) were at uncontrolled status. Mean changes in CU-QoL (36.5+/-2.7 vs 20.6+/-4.3, P=0.017) and UAS (-7.9+/-0.8 vs -3.0+/-1.0, P=0.001) were significantly different between groups I and II. The presence of serum autoantibodies and aspirin intolerance had no influence on the control of urticaria in this study. However, ASST positivity was identified as a significant predictor of CU control in multivariate analysis (OR=6.106, P=0.017).
CONCLUSIONS
The proportion of CSU patients that achieved remission or a well-controlled state was 39% for the 6 months of stepwise treatment. Longer observations are necessary to assess the exact prognosis of CSU. ASST results may be a useful parameter for predicting a better response to treatment and both UAS and CU-QoL are helpful to monitor therapeutic response.
MeSH Terms
Figure
Cited by 3 articles
-
Autologous serum and plasma skin test to predict 2-year outcome in chronic spontaneous urticaria
Tadech Boonpiyathad, Atik Sangasapaviliya
Asia Pac Allergy. 2016;6(4):226-235. doi: 10.5415/apallergy.2016.6.4.226.Clinical Course of Chronic Spontaneous Urticaria in the Korean Adult Population
Yoon Seob Kim, Sang Hyun Park, Kyungdo Han, Ji Hyun Lee, Nack In Kim, Joo Young Roh, Seong Jun Seo, Hae Jun Song, Min-Geol Lee, Jee Ho Choi, Young Min Park
Allergy Asthma Immunol Res. 2018;10(1):83-87. doi: 10.4168/aair.2018.10.1.83.Factors associated with the treatment of chronic spontaneous urticaria in children
Sun-Young Cho, Yun-Chang Choi, Byoung-Gwon Kim, Jin-A Jung
Allergy Asthma Respir Dis. 2017;5(4):211-216. doi: 10.4168/aard.2017.5.4.211.
Reference
-
1. Saini SS. Chronic spontaneous urticaria: etiology and pathogenesis. Immunol Allergy Clin North Am. 2014; 34:33–52.2. Maurer M, Weller K, Bindslev-Jensen C, Giménez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy. 2011; 66:317–330.3. O'Donnell BF. Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin North Am. 2014; 34:89–104.4. Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014; 69:868–887.5. Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014; 133:1270–1277.6. Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000; 105:664–672.7. Gaig P, Olona M, Muñoz Lejarazu D, Caballero MT, Domínguez FJ, Echechipia S, et al. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004; 14:214–220.8. Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001; 45:387–391.9. Hiragun M, Hiragun T, Mihara S, Akita T, Tanaka J, Hide M. Prognosis of chronic spontaneous urticaria in 117 patients not controlled by a standard dose of antihistamine. Allergy. 2013; 68:229–235.10. Losol P, Yoo HS, Park HS. Molecular genetic mechanisms of chronic urticaria. Allergy Asthma Immunol Res. 2014; 6:13–21.11. Toubi E, Kessel A, Avshovich N, Bamberger E, Sabo E, Nusem D, et al. Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy. 2004; 59:869–873.12. Sabroe RA, Grattan CE, Francis DM, Barr RM, Kobza Black A, Greaves MW. The autologous serum skin test: a screening test for autoantibodies in chronic idiopathic urticaria. Br J Dermatol. 1999; 140:446–452.13. Rabelo-Filardi R, Daltro-Oliveira R, Campos RA. Parameters associated with chronic spontaneous urticaria duration and severity: a systematic review. Int Arch Allergy Immunol. 2013; 161:197–204.14. Ye YM, Park JW, Kim SH, Choi JH, Hur GY, Lee HY, et al. Clinical evaluation of the computerized chronic urticaria-specific quality of life questionnaire in Korean patients with chronic urticaria. Clin Exp Dermatol. 2012; 37:722–728.15. Ye YM, Jin HJ, Hwang EK, Nam YH, Kim JH, Shin YS, et al. Co-existence of chronic urticaria and metabolic syndrome: clinical implications. Acta Derm Venereol. 2013; 93:156–160.16. Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM, et al. EAACI/GA(2)LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009; 64:1427–1443.17. Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P, Grattan CE. EAACI/GA(2)LEN task force consensus report: the autologous serum skin test in urticaria. Allergy. 2009; 64:1256–1268.18. Ye YM, Hur GY, Park HJ, Kim SH, Kim HM, Park HS. Association of specific IgE to staphylococcal superantigens with the phenotype of chronic urticaria. J Korean Med Sci. 2008; 23:845–851.19. Sahiner UM, Civelek E, Tuncer A, Yavuz ST, Karabulut E, Sackesen C, et al. Chronic urticaria: etiology and natural course in children. Int Arch Allergy Immunol. 2011; 156:224–230.20. van der Valk PG, Moret G, Kiemeney LA. The natural history of chronic urticaria and angioedema in patients visiting a tertiary referral centre. Br J Dermatol. 2002; 146:110–113.21. Kasperska-Zajac A, Sztylc J, Machura E, Jop G. Plasma IL-6 concentration correlates with clinical disease activity and serum C-reactive protein concentration in chronic urticaria patients. Clin Exp Allergy. 2011; 41:1386–1391.22. Rorsman H. Basopenia in urticaria. Acta Allergol. 1961; 16:185–215.23. Grattan CE, Dawn G, Gibbs S, Francis DM. Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin Exp Allergy. 2003; 33:337–341.24. Ye YM, Yang EM, Yoo HS, Shin YS, Kim SH, Park HS. Increased level of basophil CD203c expression predicts severe chronic urticaria. J Korean Med Sci. 2014; 29:43–47.25. Fusari A, Colangelo C, Bonifazi F, Antonicelli L. The autologous serum skin test in the follow-up of patients with chronic urticaria. Allergy. 2005; 60:256–258.26. Kulthanan K, Jiamton S, Gorvanich T, Pinkaew S. Autologous serum skin test in chronic idiopathic urticaria: prevalence, correlation and clinical implications. Asian Pac J Allergy Immunol. 2006; 24:201–206.27. Nettis E, Dambra P, D'Oronzio L, Cavallo E, Loria MP, Fanelli M, et al. Reactivity to autologous serum skin test and clinical features in chronic idiopathic urticaria. Clin Exp Dermatol. 2002; 27:29–31.28. Caproni M, Volpi W, Giomi B, Cardinali C, Antiga E, Melani L, et al. Chronic idiopathic and chronic autoimmune urticaria: clinical and immunopathological features of 68 subjects. Acta Derm Venereol. 2004; 84:288–290.29. Vohra S, Sharma NL, Mahajan VK, Shanker V. Clinicoepidemiologic features of chronic urticaria in patients having positive versus negative autologous serum skin test: a study of 100 Indian patients. Indian J Dermatol Venereol Leprol. 2011; 77:156–159.30. Kocatürk E, Kavala M, Kural E, Sarıgul S, Zındancı I. Autologous serum skin test vs autologous plasma skin test in patients with chronic urticaria: evaluation of reproducibility, sensitivity and specificity and relationship with disease activity, quality of life and anti-thyroid antibodies. Eur J Dermatol. 2011; 21:339–343.31. Staubach P, Onnen K, Vonend A, Metz M, Siebenhaar F, Tschentscher I, et al. Autologous whole blood injections to patients with chronic urticaria and a positive autologous serum skin test: a placebo-controlled trial. Dermatology. 2006; 212:150–159.32. Sabroe RA, Greaves MW. Chronic idiopathic urticaria with functional autoantibodies: 12 years on. Br J Dermatol. 2006; 154:813–819.33. Kawakami T, Kashiwakura J, Kawakami Y. Histamine-releasing factor and immunoglobulins in asthma and allergy. Allergy Asthma Immunol Res. 2014; 6:6–12.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Significance of Thyroid Autoantibodies in Urticaria
- A Study on Clinical and Etiological Aspects of Chronic Urticaria by Questionnaire
- Clinical Predictors of Disease Progression in New-Onset Urticaria
- Dermographism ( III ): Dermographism in Acute and Chronic Urticaria
- Treatment with biological products for chronic urticaria